Staff profile
Professor Jonathan Steed
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42085 |
| Fellow of the Wolfson Research Institute for Health and Wellbeing | +44 (0) 191 33 42085 |
Biography
Jonathan W. Steed was born in London, UK in 1969. He obtained his B.Sc. and Ph.D. degrees at University College London, working with Derek Tocher on organometallic and coordination chemistry. He graduated in 1993 winning the Ramsay Medal for his Ph.D. work. Between 1993 and 1995 he was a NATO postdoctoral fellow at the University of Alabama and University of Missouri, working with Jerry Atwood. In 1995 he was appointed as a Lecturer at King's College London. In 2004 he joined Durham University where he is currently Professor of Inorganic Chemistry. Professor Steed is co-author of the textbooks Supramolecular Chemistry (2000, 2009 & 2022) Core Concepts in Supramolecular Chemistry and Nanochemistry (2007) and around 400 research papers. He has edited the Encyclopaedia of Supramolecular Chemistry (2004), Organic Nanostructures (2008) and the 8-volume Supramolecular Chemistry from Molecules to Nanomaterials (2012). He is the recipient of the RSC Meldola Medal (1998), Durham's Vice Chancellor's Award for Excellence in Postgraduate Teaching (2006), the Bob Hay Lectureship (2008), the RSC Corday-Morgan Prize (2010), the RSCV Tilden Prize (2021) and the International Medal of Merit in Inclusion Compounds (2023). He is Editor-in-Chief of the American Chemical Society journal Crystal Growth & Design. His interests are in crystallization, supramolecular gels and crystalline solids particularly pharmaceutical solids, co-crystals and hydrates. See personal web pages for full details.
Supramolecular Chemistry
Traditional molecular chemistry is largely concerned with the synthesis and study of molecules linked by covalent bonds between atoms. However, there is another entire area of chemistry, often impinging on nanometre scale assemblies, that transcends the chemistry of the covalent bond. This is termed Supramolecular Chemistry and it involves the study of systems bonded by a multitude of non-covalent interactions, particularly hydrogen bonding, π-π stacking, and metal-ligand dative bonds. Many of these kinds of interactions are difficult to control yet their importance and potential is mind blowing. For example, in biochemistry Nature relies heavily on just these interactions to fold proteins into their active conformations and, crucially, it is hydrogen bonding (base pairing) and π-π stacking that give DNA its characteristic double helical shape. Prof. Steed is the author of a definitive book on Supramolecular Chemistry.1
Molecular Sensors
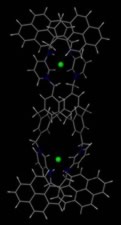
Our work encompasses many aspects of supramolecular chemistry from crystallization and the nature of individual interactions (particularly in the solid state) to their incorporation and use in functioning molecular devices, particularly in applications such as the design and synthesis of molecular sensors for anions (e.g. environmental pollutants). To take just one example, a complex molecular device based on a calixarene (shown on the right) is capable of selectively recognising and binding a two halide anions entirely through non-covalent interactions (NH···X and CH···X hydrogen bonds) and photochemically signalling that binding via the appended pyrene units.2
Supramolecular Gels

Gels comprise a liquid trapped by a highly porous network of nanometre-scale fibres. As well as being fascinating because of their complex and emergent microstructure, the highly porous, locally ordered network in gels, coupled with their formation by spontaneous self-organization gives them tremendous technological possibilities, for example in the controlled formation of highly porous polymers and in the controlled crystallization of targeted pharmaceutical polymorphic forms, an area of particular interest for our group.5 Extremely simple, readily prepared bis(urea) building blocks give gels via a hierarchical series of self-organization steps strongly influenced (both positively and negatively) by ionic additives such as metal salts. The intrinsic ability of bis(ureas) to aggregate via NH∙∙∙O=C hydrogen bonded interactions is modulated and can be switched on and of by reversible coordination interactions to metal cations and hydrogen bonding to conjugate anions. Our group are particularly active in the design of targeted gels for pharmaceutical crystal form control. An SEM image of a dried gel showing chiral helical fibres derived from a chiral gelator is shown left.3-7
Crystallography and Solid Form
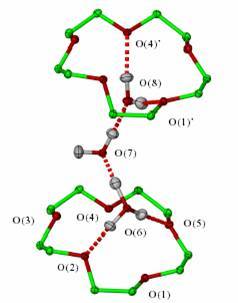
Facilities at Durham for both single crystal and powder work are internationally leading. The group is also particularly active in structure determination by neutron diffraction and students have the opportunity of taking part in visits to facilities at the ILL in Grenoble, France or the ISIS facility in the UK. Our single crystal neutron structure of the exotic H7O3+ ion trapped by two molecules called 'crown ethers' is shown right. We are particularly interested in low symmetry crystal structures with more than one molecule in the asymmetric unit7 and we maintain a dedicated web resource on this work (http://zprime.co.uk). The group are also expert in the study of polymorphism, particularly in pharmaceutical hydrates and in the use of novel methods such as gel phase crystal growth and mechanochemistry to influence solid form.
References
- J. W. Steed and J. L. Atwood, "Supramolecular Chemistry", 3rd Ed, J. Wiley & Sons: Chichester, 2022.
- “Induced Fit Inter-Anion Discrimination by Binding-Induced Excimer Formation”, M. H. Filby, S. J. Dickson, N. Zaccheroni, L. Prodi, S. Bonacchi, M. Montalti, M. J. Paterson, T. D. Humphries, C. Chiorboli, and J. W. Steed, J. Am. Chem. Soc., 2008, 130, 4105-4113.
- "Anion-Tuning of Supramolecular Gel Properties", G. O. Lloyd and J. W. Steed, Nature Chem., 2009, 1, 437.
- "Metal- and Anion Binding Supramolecular Gels", M. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960.
- "Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth”, J. A. Foster, M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke, J. A. K. Howard and J. W. Steed, Nature Chem., 2010, 2, 1037–1043.
- “Halogen-Bonding-Triggered Supramolecular Gel Formation”, L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nature Chem., 2013, 5, 42–47.
- “Blending gelators to tune gel properties and probe anion-induced disassembly”, J. A. Foster, R. M. Edkins, G. J. Cameron, N. Colgin, K. Fucke, S. Ridgeway, A. G. Crawford, T. B. Marder, A. Beeby, S. L. Cobb and J. W. Steed, Chem. Eur. J., 2014, 20, 279–291.
- “Packing Problems: High Z′ Crystal Structures and their Relationship to Cocrystals, Inclusion Compounds and Polymorphism,” K. M. Steed and J. W. Steed, Chem. Rev. 2015, 115, 2895-2933.
Research interests
- Supramolecular Chemistry
- Crystallography
- X-ray Diffraction
- Molecular Materials
- Gels
- Pharmaceutical Solids
- Pharmaceuticals Analysis
Esteem Indicators
- 2021: Tilden Prize: Royal Society of Chemistry Tilden Prize
Publications
Journal Article
- Selective crystallization of pyrazinamide polymorphs in supramolecular gels: Synergistic selectivity by mimetic gelator and solventZhang, Q., Yan, Y., Xu, Y., Zhang, X., & Steed, J. W. (2025). Selective crystallization of pyrazinamide polymorphs in supramolecular gels: Synergistic selectivity by mimetic gelator and solvent. Journal of Colloid and Interface Science, 687, 582-588. https://doi.org/10.1016/j.jcis.2025.02.093
- Designing lenalidomide cocrystals with an extended-release profile for improved pulmonary drug deliveryScreen, M. A., Tomkinson, G., McCabe, J. F., Askin, S., Mahon, C. S., Wilson, M. R., & Steed, J. W. (2025). Designing lenalidomide cocrystals with an extended-release profile for improved pulmonary drug delivery. New Journal of Chemistry, 49(16), 6535-6543. https://doi.org/10.1039/d5nj00425j
- A tailored graphene supramolecular gel for pharmaceutical crystallizationZhang, Q., Screen, M. A., Bowen, L., Xu, Y., Zhang, X., & Steed, J. W. (2025). A tailored graphene supramolecular gel for pharmaceutical crystallization. Chemical Science. Advance online publication. https://doi.org/10.1039/d4sc08087d
- Intercalation of Neutral Guests in Pillared Salt Cocrystals of 5‑Ureidosalyclic AcidKennedy, S. R., Blundell, T. J., Henderson, E. F., Miquelot, A. P., & Steed, J. W. (2025). Intercalation of Neutral Guests in Pillared Salt Cocrystals of 5‑Ureidosalyclic Acid. Crystal Growth and Design, 25(5), 1614-1621. https://doi.org/10.1021/acs.cgd.4c01715
- Netting Crystal Nuclei in Metal–Organic Framework CavitiesBraschinsky, A., Blundell, T. J., & Steed, J. W. (2024). Netting Crystal Nuclei in Metal–Organic Framework Cavities. Small Structures, 5(12), Article 2400300. https://doi.org/10.1002/sstr.202400300
- Supramolecular gels: a versatile crystallization toolboxContreras-Montoya, R., Álvarez de Cienfuegos, L., Gavira, J. A., & Steed, J. W. (2024). Supramolecular gels: a versatile crystallization toolbox. Chemical Society Reviews, 21, 10604-10619. https://doi.org/10.1039/d4cs00271g
- Crystalline Molecular Cleft ClathratesLynch, A. V., Blundell, T. J., & Steed, J. W. (2024). Crystalline Molecular Cleft Clathrates. Crystal Growth & Design, 24(17), 7271-7277. https://doi.org/10.1021/acs.cgd.4c00928
- What Has Carbamazepine Taught Crystal Engineers?Hall, A. V., Cruz-Cabeza, A. J., & Steed, J. W. (2024). What Has Carbamazepine Taught Crystal Engineers?. Crystal Growth and Design, 24(17), 7342-7360. https://doi.org/10.1021/acs.cgd.4c00555
- Scrolling in Supramolecular Gels: A Designer’s GuideJones, C. D., Kershaw Cook, L. J., Slater, A. G., Yufit, D. S., & Steed, J. W. (2024). Scrolling in Supramolecular Gels: A Designer’s Guide. Chemistry of Materials, 36(6), 2799-2809. https://doi.org/10.1021/acs.chemmater.3c03013
- Unprecedented light induced aggregation of cationic 1,4,5,8-naphthalenediimide amphiphilesAntoneli, R. G., Moraes, T. B. F., Junqueira, H. C., Sihn, L. M., Toma, H. E., Pedras, B., Ferreira, L. F. V., Frath, D., Bucher, C., Steed, J. W., Demets, G. J., & Triboni, E. R. (2024). Unprecedented light induced aggregation of cationic 1,4,5,8-naphthalenediimide amphiphiles. Journal of Materials Chemistry C, 12(11), 3888-3896. https://doi.org/10.1039/d3tc04178f
- Pushing Technique Boundaries to Probe Conformational Polymorphism.Ward, M. R., Taylor, C. R., Mulvee, M. T., Lampronti, G. I., Belenguer, A. M., Steed, J. W., Day, G. M., & Oswald, I. D. H. (2023). Pushing Technique Boundaries to Probe Conformational Polymorphism. Crystal Growth and Design, 23(10), 7217-7230. https://doi.org/10.1021/acs.cgd.3c00641
- Pathway complexity in fibre assembly: from liquid crystals to hyper-helical gelmorphs.Contreras-Montoya, R., Smith, J. P., Boothroyd, S. C., Aguilar, J. A., Mirzamani, M., Screen, M. A., Yufit, D. S., Robertson, M., He, L., Qian, S., Kumari, H., Steed, J. W., Contreras-Montoya, R., Smith, J. P., Boothroyd, S. C., Mirzamani, M., Screen, M. A., Yufit, D. S., Robertson, M., & He, L. (2023). Pathway complexity in fibre assembly: from liquid crystals to hyper-helical gelmorphs. Chemical Science, 14(41), 11389-11401. https://doi.org/10.1039/d3sc03841f
- Vapor Sorption and Halogen-Bond-Induced Solid-Form Rearrangement of a Porous PharmaceuticalAndrews, J. L., Yufit, D. S., McCabe, J. F., Fox, M. A., & Steed, J. W. (2023). Vapor Sorption and Halogen-Bond-Induced Solid-Form Rearrangement of a Porous Pharmaceutical. Crystal Growth & Design, 23(4), 2628-2633. https://doi.org/10.1021/acs.cgd.2c01464
- Metal-based gels: Synthesis, properties, and applicationsPicci, G., Caltagirone, C., Garau, A., Lippolis, V., Milia, J., & Steed, J. W. (2023). Metal-based gels: Synthesis, properties, and applications. Coordination Chemistry Reviews, 492. https://doi.org/10.1016/j.ccr.2023.215225
- Molecular clusters in confined spacesBraschinsky, A., & Steed, J. W. (2022). Molecular clusters in confined spaces. Coordination Chemistry Reviews, 473. https://doi.org/10.1016/j.ccr.2022.214840
- Designer Gelators for the Crystallization of a Salt Active Pharmaceutical Ingredient─Mexiletine HydrochlorideAndrews, J. L., Kennedy, S. R., Yufit, D. S., McCabe, J. F., & Steed, J. W. (2022). Designer Gelators for the Crystallization of a Salt Active Pharmaceutical Ingredient─Mexiletine Hydrochloride. Crystal Growth and Design, 22(11), 6775-6785. https://doi.org/10.1021/acs.cgd.2c00925
- Integral Role of Water in the Solid-State Behavior of the Antileishmanial Drug MiltefosineHall, A. V., Gostick, I. E., Yufit, D. S., Marchant, G. Y., Kirubakaran, P., Madu, S. J., Li, M., Steel, P. G., & Steed, J. W. (2022). Integral Role of Water in the Solid-State Behavior of the Antileishmanial Drug Miltefosine. Crystal Growth and Design, 22(10), 6262-6266. https://doi.org/10.1021/acs.cgd.2c00843
- Prediction and Preparation of Coamorphous Phases of a BislactamChambers, L. I., Musa, O. M., & Steed, J. W. (2022). Prediction and Preparation of Coamorphous Phases of a Bislactam. Molecular Pharmaceutics, 19(7), 2651-2661. https://doi.org/10.1021/acs.molpharmaceut.2c00357
- The “Magic Linker”: Highly Effective Gelation from Sterically Awkward PackingSmith, J. P., Yufit, D. S., McCabe, J. F., & Steed, J. W. (2022). The “Magic Linker”: Highly Effective Gelation from Sterically Awkward Packing. Crystal Growth and Design, 22(3), 1914-1921. https://doi.org/10.1021/acs.cgd.1c01470
- Biofunctionality with a twist: the importance of molecular organisation, handedness and configuration in synthetic biomaterial designHendrikse, S. I., Contreras-Montoya, R., Ellis, A. V., Thordarson, P., & Steed, J. W. (2022). Biofunctionality with a twist: the importance of molecular organisation, handedness and configuration in synthetic biomaterial design. Chemical Society Reviews, 51(1), 28-42. https://doi.org/10.1039/d1cs00896j
- Structure and hydration of polyvinylpyrrolidone-hydrogen peroxideChambers, L. I., Yufit, D. S., Fox, M. A., Musa, O. M., & Steed, J. W. (2022). Structure and hydration of polyvinylpyrrolidone-hydrogen peroxide. Chemical Communications, 58(1), 80-83. https://doi.org/10.1039/d1cc06047c
- Understanding the Interaction of Gluconamides and Gluconates with Amino Acids in Hair CareChambers, L. I., Yufit, D. S., Musa, O. M., & Steed, J. W. (2022). Understanding the Interaction of Gluconamides and Gluconates with Amino Acids in Hair Care. Crystal Growth and Design, 22(10), 6190-6200. https://doi.org/10.1021/acs.cgd.2c00753
- Anion-Responsive Fluorescent Supramolecular GelsPicci, G., Mulvee, M. T., Caltagirone, C., Lippolis, V., Frontera, A., Gomila, R. M., & Steed, J. W. (2022). Anion-Responsive Fluorescent Supramolecular Gels. Molecules, 27(4), Article 1257. https://doi.org/10.3390/molecules27041257
- Highly Thermally Resistant Bisamide Gelators as Pharmaceutical Crystallization MediaTorres-Moya, I., Sánchez, A., Saikia, B., Yufit, D. S., Prieto, P., Carrillo, J. R., & Steed, J. W. (2022). Highly Thermally Resistant Bisamide Gelators as Pharmaceutical Crystallization Media. Gels, 9(1), Article 26. https://doi.org/10.3390/gels9010026
- Derisking the Polymorph Landscape: The Complex Polymorphism of Mexiletine HydrochlorideAndrews, J. L., Nilsson Lill, S. O., Freitag-Pohl, S., Apperley, D. C., Yufit, D. S., Batsanov, A. S., Mulvee, M. T., Edkins, K., McCabe, J. F., Berry, D. J., Probert, M. R., & Steed, J. W. (2021). Derisking the Polymorph Landscape: The Complex Polymorphism of Mexiletine Hydrochloride. Crystal Growth & Design, 21(12), 7150-7167. https://doi.org/10.1021/acs.cgd.1c01009
- Enhancement of sensitivity for dichlorvos detection by a low-weight gelator based on bolaamphiphile amino acid derivatives decorated with a hybrid graphene quantum dot/enzyme/hydrogelSahub, C., Andrews, J. L., Smith, J. P., Mohamad Arif, M. A., Tomapatanaget, B., & Steed, J. W. (2021). Enhancement of sensitivity for dichlorvos detection by a low-weight gelator based on bolaamphiphile amino acid derivatives decorated with a hybrid graphene quantum dot/enzyme/hydrogel. Materials Chemistry Frontiers, 5(18), 6850-6859. https://doi.org/10.1039/d1qm00296a
- Crystal Habit Modification of Metronidazole by Supramolecular Gels with Complementary FunctionalityJayabhavan, S. S., Steed, J. W., & Damodaran, K. K. (2021). Crystal Habit Modification of Metronidazole by Supramolecular Gels with Complementary Functionality. Crystal Growth & Design, 21(9), 5383-5393. https://doi.org/10.1021/acs.cgd.1c00659
- Properties and Applications of Stimuli-Responsive DiacetylenesHall, A. V., Musa, O. M., & Steed, J. W. (2021). Properties and Applications of Stimuli-Responsive Diacetylenes. Crystal Growth and Design, 21(6), 3614-3638. https://doi.org/10.1021/acs.cgd.1c00300
- High-Yielding Flow Synthesis of a Macrocyclic Molecular HingeJones, C. D., Kershaw Cook, L. J., Marquez-Gamez, D., Luzyanin, K. V., Steed, J. W., & Slater, A. G. (2021). High-Yielding Flow Synthesis of a Macrocyclic Molecular Hinge. Journal of the American Chemical Society, 143(19), 7553-7565. https://doi.org/10.1021/jacs.1c02891
- Alkali Metal Salts of 10,12-Pentacosadiynoic Acid and Their Dosimetry ApplicationsHall, A. V., Musa, O. M., Hood, D. K., Apperley, D. C., Yufit, D. S., & Steed, J. W. (2021). Alkali Metal Salts of 10,12-Pentacosadiynoic Acid and Their Dosimetry Applications. Crystal Growth and Design, 21(4), 2416-2422. https://doi.org/10.1021/acs.cgd.1c00031
- Predictive identification of co-formers in co-amorphous systemsChambers, L. I., Grohganz, H., Palmelund, H., Löbmann, K., Rades, T., Musa, O. M., & Steed, J. W. (2021). Predictive identification of co-formers in co-amorphous systems. European Journal of Pharmaceutical Sciences, 157, Article 105636. https://doi.org/10.1016/j.ejps.2020.105636
- Anisotropic thermal expansion effects in layered n-Alkyl carboxylic acid – bipyridyl cocrystalsHall, A. V., Yufit, D. S., Zhang, Y., Musa, O. M., & Steed, J. W. (2021). Anisotropic thermal expansion effects in layered n-Alkyl carboxylic acid – bipyridyl cocrystals. Supramolecular Chemistry, 33(10). https://doi.org/10.1080/10610278.2022.2117623
- Guest inclusion by Borromean weave coordination networksByrne, P., Lloyd, G. O., & Steed, J. W. (2021). Guest inclusion by Borromean weave coordination networks. Journal of Coordination Chemistry, 74(1-3), 152-161. https://doi.org/10.1080/00958972.2021.1875132
- Minimizing polymorphic risk through cooperative computational and experimental explorationTaylor, C. R., Mulvee, M. T., Perenyi, D. S., Probert, M. R., Day, G. M., & Steed, J. W. (2020). Minimizing polymorphic risk through cooperative computational and experimental exploration. Journal of the American Chemical Society, 142(39), 16668-16680. https://doi.org/10.1021/jacs.0c06749
- The crystal engineering of radiation-sensitive diacetylene cocrystals and saltsHall, A. V., Yufit, D. S., Apperley, D. C., Senak, L., Musa, O. M., Hood, D. K., & Steed, J. W. (2020). The crystal engineering of radiation-sensitive diacetylene cocrystals and salts. Chemical Science, 11(30), 8025-8035. https://doi.org/10.1039/d0sc02540b
- Lilypad aggregation: localised self-assembly and metal sequestration at a liquid-vapour interfaceJones, C. D., Lewis, A. R., Jones, D. R., Ottley, C., Liu, K., & Steed, J. W. (2020). Lilypad aggregation: localised self-assembly and metal sequestration at a liquid-vapour interface. Chemical Science, 11(28), 7501-7510. https://doi.org/10.1039/d0sc02190c
- Encapsulated Nanodroplet Crystallization of Organic-Soluble Small MoleculesTyler, A. R., Ragbirsingh, R., McMonagle, C. J., Waddell, P. G., Heaps, S. E., Steed, J. W., Thaw, P., Hall, M. J., & Probert, M. R. (2020). Encapsulated Nanodroplet Crystallization of Organic-Soluble Small Molecules. Chem, 6(7), 1755-1765. https://doi.org/10.1016/j.chempr.2020.04.009
- Hybrid GMP–polyamine hydrogels as new biocompatible materials for drug encapsulationLopera, A., Aguilar, J. A., Belda, R., Verdejo, B., Steed, J. W., & García-España, E. (2020). Hybrid GMP–polyamine hydrogels as new biocompatible materials for drug encapsulation. Soft Matter, 16(28), 6514-6522. https://doi.org/10.1039/d0sm00704h
- Calcium cyclic carboxylates as structural models for calcium carbonate scale inhibitorsHong, Y., Yufit, D. S., Letzelter, N., & Steed, J. W. (2020). Calcium cyclic carboxylates as structural models for calcium carbonate scale inhibitors. CrystEngComm, 22(15), 2585-2592. https://doi.org/10.1039/d0ce00243g
- Drug Mimetic Organogelators for the Control of Concomitant Crystallization of Barbital and ThalidomideSaikia, B., Mulvee, M. T., Torres-Moya, I., Sarma, B., & Steed, J. W. (2020). Drug Mimetic Organogelators for the Control of Concomitant Crystallization of Barbital and Thalidomide. Crystal Growth and Design, 20(12), 7989-7996. https://doi.org/10.1021/acs.cgd.0c01240
- Threaded Rings that Swim in Excitable MediaCincotti, A., Maucher, F., Evans, D., Chapin, B. M., Horner, K., Bromley, E., Lobb, A., Steed, J. W., & Sutcliffe, P. (2019). Threaded Rings that Swim in Excitable Media. Physical Review Letters, 123(25), Article 258102. https://doi.org/10.1103/physrevlett.123.258102
- Extensive sequential polymorphic interconversion in the solid-state: Two hydrates and ten anhydrous phases of hexamidine diisethionateEdkins, K., McIntyre, G. J., Wilkinson, C., Kahlenberg, V., Toebbens, D. M., Griesser, U. J., Bruening, J., Schmidt, M. U., & Steed, J. W. (2019). Extensive sequential polymorphic interconversion in the solid-state: Two hydrates and ten anhydrous phases of hexamidine diisethionate. Crystal Growth and Design, 2019(19), 7280-7289. https://doi.org/10.1021/acs.cgd.9b01170
- Carbohydrate-Supramolecular Gels: Adsorbents for Chromium(VI) Removal from WastewaterRizzo, C., Andrews, J. L., Steed, J. W., & D’Anna, F. (2019). Carbohydrate-Supramolecular Gels: Adsorbents for Chromium(VI) Removal from Wastewater. Journal of Colloid and Interface Science, 548, 184-196. https://doi.org/10.1016/j.jcis.2019.04.034
- High thermal stability, pH responsive organogels of 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization mediaTorres-Moya, I., Saikia, B., Prieto, P., Carrillo, J. R., & Steed, J. W. (2019). High thermal stability, pH responsive organogels of 2H-benzo[d]1,2,3-triazole derivatives as pharmaceutical crystallization media. CrystEngComm, 21(13), 2135-2143. https://doi.org/10.1039/c8ce01742e
- Bacterial sensors define intracellular free energies for correct enzyme metalationOsman, D., Martini, M. A., Foster, A. W., Chen, J., Scott, A. J., Morton, R. J., Steed, J. W., Lurie-Luke, E., Huggins, T. G., Lawrence, A. D., Deery, E., Warren, M. J., Chivers, P. T., & Robinson, N. J. (2019). Bacterial sensors define intracellular free energies for correct enzyme metalation. Nature Chemical Biology, 15(3), 241-249. https://doi.org/10.1038/s41589-018-0211-4
- Braiding, branching and chiral amplification of nanofibres in supramolecular gelsJones, C. D., Simmons, H. T., Horner, K. E., Liu, K., Thompson, R. L., & Steed, J. W. (2019). Braiding, branching and chiral amplification of nanofibres in supramolecular gels. Nature Chemistry, 11, 375-381. https://doi.org/10.1038/s41557-019-0222-0
- 21st Century Developments in the Understanding and Control of Molecular SolidsSteed, J. W. (2018). 21st Century Developments in the Understanding and Control of Molecular Solids. Chemical Communications, 54(94), 13175-13182. https://doi.org/10.1039/c8cc08277d
- Crystal habit modification of Cu(ii) isonicotinate–N-oxide complexes using gel phase crystallisationGhosh, D., Ferfolja, K., Drabavičius, Žygimantas, Steed, J. W., & Damodaran, K. K. (2018). Crystal habit modification of Cu(ii) isonicotinate–N-oxide complexes using gel phase crystallisation. New Journal of Chemistry, 42(24), 19963-19970. https://doi.org/10.1039/c8nj05036h
- Investigating the effect of supramolecular gel phase crystallization on gel nucleationDawn, A., Mirzamani, M., Jones, C. D., Yufit, D. S., Qian, S., Steed, J. W., & Kumari, H. (2018). Investigating the effect of supramolecular gel phase crystallization on gel nucleation. Soft Matter, 14(46), 9489-9497. https://doi.org/10.1039/c8sm01916a
- Supramolecular gelation as the first stage in Ostwald’s ruleAndrews, J. L., Pearson, E., Yufit, D. S., Steed, J. W., & Edkins, K. (2018). Supramolecular gelation as the first stage in Ostwald’s rule. Crystal Growth and Design, 18(12), 7690-7700. https://doi.org/10.1021/acs.cgd.8b01539
- Gelation by histidine-derived ureasCoubrough, H. M., Jones, C. D., Yufit, D. S., & Steed, J. W. (2018). Gelation by histidine-derived ureas. Supramolecular Chemistry, 30(5-6), 384-394. https://doi.org/10.1080/10610278.2017.1376062
- Dynamic behaviour in nicotinate-bridged binuclear ruthenium(IV) complexesSteed, J. W., & Tocher, D. A. (2018). Dynamic behaviour in nicotinate-bridged binuclear ruthenium(IV) complexes. Polyhedron, 147, 152-155. https://doi.org/10.1016/j.poly.2018.03.025
- Structures of a Clathrate Hydrate Former, Inhibitor, and Synergist in WaterPerrin, A., Goodwin, M. J., Callear, S., Soper, A. K., Musa, O. M., & Steed, J. W. (2018). Structures of a Clathrate Hydrate Former, Inhibitor, and Synergist in Water. Journal of Physical Chemistry B (Soft Condensed Matter and Biophysical Chemistry), 122(18), 4901-4912. https://doi.org/10.1021/acs.jpcb.8b02762
- Activating [4+4] photoreactivity in the solid-state via complexation: from 9-(methylaminomethyl)anthracene to its silver(I) complexesSpinelli, F., d’Agostino, S., Taddei, P., Jones, C. D., Steed, J. W., & Grepioni, F. (2018). Activating [4+4] photoreactivity in the solid-state via complexation: from 9-(methylaminomethyl)anthracene to its silver(I) complexes. Dalton Transactions, 16(47), 5725-5733. https://doi.org/10.1039/c8dt00198g
- Tailored Supramolecular Gel and Microemulsion Crystallization Strategies – is Isoniazid Really Monomorphic?Kennedy, S. R., Jones, C. D., Yufit, D. S., Nicholson, C. E., Cooper, S. J., & Steed, J. W. (2018). Tailored Supramolecular Gel and Microemulsion Crystallization Strategies – is Isoniazid Really Monomorphic?. CrystEngComm, 20(10), 1390-1398. https://doi.org/10.1039/c8ce00066b
- Phosphate-Free Inhibition of Calcium Carbonate Dishwasher DepositsHong, Y., Letzelter, N., Evans, J. S., Yufit, D. S., & Steed, J. W. (2018). Phosphate-Free Inhibition of Calcium Carbonate Dishwasher Deposits. Crystal Growth and Design, 18(3), 1526-1538. https://doi.org/10.1021/acs.cgd.7b01508
- Small-Molecule Povidone Analogs in Coamorphous Pharmaceutical PhasesGoodwin, M., Musa, O., Berry, D., & Steed, J. W. (2018). Small-Molecule Povidone Analogs in Coamorphous Pharmaceutical Phases. Crystal Growth and Design, 18(2), 701-709. https://doi.org/10.1021/acs.cgd.7b01062
- Boric Acid Co-crystals in Guar GelationPerrin, A., Goodwin, M. J., Musa, O. M., Yufit, D. S., & Steed, J. W. (2017). Boric Acid Co-crystals in Guar Gelation. CrystEngComm, 19(47), 7125-7131. https://doi.org/10.1039/c7ce01858d
- Scrolling of supramolecular lamellae in the hierarchical self-assembly of fibrous gelsJones, C. D., Kennedy, S. R., Walker, M., Yufit, D. S., & Steed, J. W. (2017). Scrolling of supramolecular lamellae in the hierarchical self-assembly of fibrous gels. Chem, 3(4), 603-628. https://doi.org/10.1016/j.chempr.2017.09.001
- Halogen and Hydrogen Bonding in Povidone-Iodine and Related Co-PhasesGoodwin, M. J., Steed, B. W., Yufit, D. S., Musa, O. M., Berry, D. J., & Steed, J. W. (2017). Halogen and Hydrogen Bonding in Povidone-Iodine and Related Co-Phases. Crystal Growth and Design, 17(10), 5552-5558. https://doi.org/10.1021/acs.cgd.7b01103
- Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based designBerry, D. J., & Steed, J. W. (2017). Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Advanced Drug Delivery Reviews, 117, 3-24. https://doi.org/10.1016/j.addr.2017.03.003
- Hydration Behavior of Polylactam Clathrate Hydrate Inhibitors and Their Small-Molecule Model CompoundsPerrin, A., Goodwin, M. J., Musa, O. M., Berry, D. J., Corner, P., Edkins, K., Yufit, D. S., & Steed, J. W. (2017). Hydration Behavior of Polylactam Clathrate Hydrate Inhibitors and Their Small-Molecule Model Compounds. Crystal Growth and Design, 17(6), 3236-3249. https://doi.org/10.1021/acs.cgd.7b00221
- GMP polyamine hybrid hydrogels: enhanced gel strength probed by z-spectroscopyAguilar, J. A., Belda, R., García-España, E., Morris, G. A., & Steed, J. W. (2017). GMP polyamine hybrid hydrogels: enhanced gel strength probed by z-spectroscopy. Chemistry - A European Journal, 23(32), 7755-7760. https://doi.org/10.1002/chem.201700642
- Supramolecular MaterialsAmabilino, D. B., Smith, D. K., & Steed, J. W. (2017). Supramolecular Materials. Chemical Society Reviews, 46(9), 2404-2420. https://doi.org/10.1039/c7cs00163k
- High Pressure/Low Temperature Polymorphism in 2,6-Dimethylpyridine–Formic Acid CocrystalsLee, R., Yufit, D. S., Probert, M. R., & Steed, J. W. (2017). High Pressure/Low Temperature Polymorphism in 2,6-Dimethylpyridine–Formic Acid Cocrystals. Crystal Growth and Design, 17(4), 1647-1653. https://doi.org/10.1021/acs.cgd.6b01656
- Metal ‘turn-off’, anion ‘turn-on’ gelation cascade in pyridinylmethyl ureasOffiler, C. A., Jones, C. D., & Steed, J. W. (2017). Metal ‘turn-off’, anion ‘turn-on’ gelation cascade in pyridinylmethyl ureas. Chemical Communications, 53(12), 2024-2027. https://doi.org/10.1039/c6cc09126a
- Pharmaceutical Polymorph Control in a Drug-Mimetic Supramolecular GelFoster, J. A., Damodaran, K. K., Maurin, A., Day, G. M., Thompson, H. P., Cameron, G. J., Bernal, J. C., & Steed, J. W. (2017). Pharmaceutical Polymorph Control in a Drug-Mimetic Supramolecular Gel. Chemical Science, 8(1), 78-84. https://doi.org/10.1039/c6sc04126d
- Neutron Diffraction Studies on Guest-Induced Distortions in Urea Inclusion CompoundsLee, R., Mason, S. A., Mossou, E., Lamming, G., Probert, M. R., & Steed, J. W. (2016). Neutron Diffraction Studies on Guest-Induced Distortions in Urea Inclusion Compounds. Crystal Growth and Design, 16(12), 7175-7185. https://doi.org/10.1021/acs.cgd.6b01371
- Trimeric Cyclamers: Hierarchical High Z′ Crystal Engineering Based on Guest Structure and BasicityKennedy, S. R., Miquelot, A., Aguilar, J. A., & Steed, J. W. (2016). Trimeric Cyclamers: Hierarchical High Z′ Crystal Engineering Based on Guest Structure and Basicity. Chemical Communications, 52(79), 11846-11849. https://doi.org/10.1039/c6cc06054d
- Gels with sense: supramolecular materials that respond to heat, light and soundJones, C. D., & Steed, J. W. (2016). Gels with sense: supramolecular materials that respond to heat, light and sound. Chemical Society Reviews, 45(23), 6546-6596. https://doi.org/10.1039/c6cs00435k
- Knot theory in modern chemistryHorner, K. E., Miller, M. A., Steed, J. W., & Sutcliffe, P. M. (2016). Knot theory in modern chemistry. Chemical Society Reviews, 45(23), 6432-6448. https://doi.org/10.1039/c6cs00448b
- Cavity-Containing Supramolecular Gels as a Crystallization Tool for Hydrophobic PharmaceuticalsKaufmann, L., Kennedy, S. R., Jones, C. D., & Steed, J. W. (2016). Cavity-Containing Supramolecular Gels as a Crystallization Tool for Hydrophobic Pharmaceuticals. Chemical Communications, 52(66), 10113-10116. https://doi.org/10.1039/c6cc04037c
- Modulating the Hydration Behaviour of Calcium Chloride by Lactam ComplexationPerrin, A., Musa, O. M., & Steed, J. W. (2016). Modulating the Hydration Behaviour of Calcium Chloride by Lactam Complexation. Dalton Transactions, 45(30), 12181-12187. https://doi.org/10.1039/c6dt02296k
- Expanding the Pyridine–Formic Acid Cocrystal Landscape under Extreme ConditionsLee, R., Firbank, A. J., Probert, M. R., & Steed, J. W. (2016). Expanding the Pyridine–Formic Acid Cocrystal Landscape under Extreme Conditions. Crystal Growth and Design, 16(7), 4005-4011. https://doi.org/10.1021/acs.cgd.6b00544
- Pharmaceutical crystallization with nanocellulose organogelsRuiz-Palomero, C., Kennedy, S. R., Soriano, M. L., Jones, C. D., Valcárcel, M., & Steed, J. W. (2016). Pharmaceutical crystallization with nanocellulose organogels. Chemical Communications, 52(50), 7782-7785. https://doi.org/10.1039/c6cc03088b
- Anion Hydrogen Bonding from a ‘Revealed’ Urea LigandQureshi, N., Yufit, D. S., Steed, K. M., Howard, J. A., & Steed, J. W. (2016). Anion Hydrogen Bonding from a ‘Revealed’ Urea Ligand. CrystEngComm, 18, 5333-5337. https://doi.org/10.1039/c6ce01039c
- Polymorphism of (Z)-3-Bromopropenoic acid: a high and low Z' pairReddy, J. P., & Steed, J. W. (2016). Polymorphism of (Z)-3-Bromopropenoic acid: a high and low Z’ pair. Crystal Growth and Design, 16, 4021-4025. https://doi.org/10.1021/acs.cgd.6b00551
- Stabilisation of an amorphous form of ROY through a predicted co-former interactionCorner, P. A., Harburn, J. J., Steed, J. W., McCabe, J. F., & Berry, D. J. (2016). Stabilisation of an amorphous form of ROY through a predicted co-former interaction. Chemical Communications, 52(39), 6537-6540. https://doi.org/10.1039/c6cc02949c
- Fluorescent carbon quantum dot hydrogels for direct determination of silver ionsCayuela, A., Soriano, M. L., Kennedy, S. R., Steed, J., & Valcárcel, M. (2016). Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta, 151, 100-105. https://doi.org/10.1016/j.talanta.2016.01.029
- Manipulating three-dimensional gel network entanglement by thin film shearingKumari, H., Kline, S. R., Kennedy, S. R., Garvey, C., Raston, C. L., Atwood, J. L., & Steed, J. W. (2016). Manipulating three-dimensional gel network entanglement by thin film shearing. Chemical Communications, 52(24), 4513-4516. https://doi.org/10.1039/c6cc00171h
- Gelation by supramolecular dimerization of mono(urea)sHooper, A. E., Kennedy, S. R., Jones, C. D., & Steed, J. W. (2016). Gelation by supramolecular dimerization of mono(urea)s. Chemical Communications, 52(1), 198-201. https://doi.org/10.1039/c5cc06995e
- Fluorous ‘Ponytails’ Lead to Strong Gelators Showing Thermally Induced Structure EvolutionKumari, H., Armitage, S. E., Kline, S. R., Damodaran, K. K., Kennedy, S. R., Atwood, J. L., & Steed, J. W. (2015). Fluorous ‘Ponytails’ Lead to Strong Gelators Showing Thermally Induced Structure Evolution. Soft Matter, 11(43), 8471-8478. https://doi.org/10.1039/c5sm01865j
- Selective gelation of N-(4-pyridyl)nicotinamide by copper(II) saltsGhosh, D., Lebedytė, I., Yufit, D. S., Damodaran, K. K., & Steed, J. W. (2015). Selective gelation of N-(4-pyridyl)nicotinamide by copper(II) salts. CrystEngComm, 17(42), 8130-8138. https://doi.org/10.1039/c5ce00901d
- Fluorescent carbon dot–molecular salt hydrogelsCayuela, A., Kennedy, S. R., Soriano, M. L., Valcárcel, M., & Steed, J. W. (2015). Fluorescent carbon dot–molecular salt hydrogels. Chemical Science, 6(11), 6139-6146. https://doi.org/10.1039/c5sc01859e
- Supramolecular Gel Control of Cisplatin Crystallization: Identification of a New Solvate Form Using a Cisplatin-Mimetic GelatorDawn, A., Andrew, K. S., Yufit, D. S., Hong, Y., Reddy, J. P., Jones, C. D., Aguilar, J. A., & Steed, J. W. (2015). Supramolecular Gel Control of Cisplatin Crystallization: Identification of a New Solvate Form Using a Cisplatin-Mimetic Gelator. Crystal Growth and Design, 15(9), 4591-4599. https://doi.org/10.1021/acs.cgd.5b00840
- The problems associated with sour gas in the oilfield industry and their solutionsGoodwin, M. J., Musa, O. M., & Steed, J. W. (2015). The problems associated with sour gas in the oilfield industry and their solutions. Energy and Fuels, 29(8), 4667-4682. https://doi.org/10.1021/acs.energyfuels.5b00952
- Packing Problems: High Z′ Crystal Structures and their Relationship to Cocrystals, Inclusion Compounds and PolymorphismSteed, K. M., & Steed, J. W. (2015). Packing Problems: High Z′ Crystal Structures and their Relationship to Cocrystals, Inclusion Compounds and Polymorphism. Chemical Reviews, 115(8), 2895-2933. https://doi.org/10.1021/cr500564z
- Conserved hydrogen bonding in tetrahydrocarbazolone derivatives: influence of solution-state assembly on crystal form nucleationEdkins, R. M., Hayden, E., Steed, J. W., & Fucke, K. (2015). Conserved hydrogen bonding in tetrahydrocarbazolone derivatives: influence of solution-state assembly on crystal form nucleation. Chemical Communications, 51(25), 5314-5317. https://doi.org/10.1039/c4cc10453f
- Insights into the Crystallisation Process from Anhydrous, Hydrated and Solvated Crystal Forms of Diatrizoic AcidFucke, K., McIntyre, G. J., Lemee-Cailleay, M., Wilkinson, C., Edwards, A. J., Howard, J. A., & Steed, J. W. (2015). Insights into the Crystallisation Process from Anhydrous, Hydrated and Solvated Crystal Forms of Diatrizoic Acid. Chemistry - A European Journal, 21(3), 1036-1047. https://doi.org/10.1002/chem.201404693
- Highly Interlocked Anion-Bridged Supramolecular Networks from Interrupted Imidazole-Urea GelsJames, S., Perrin, A., Jones, C., Yufit, D., & Steed, J. (2014). Highly Interlocked Anion-Bridged Supramolecular Networks from Interrupted Imidazole-Urea Gels. Chemical Communications, 50(85), 12851-12854. https://doi.org/10.1039/c4cc05789a
- Hydrogen Bonding Effects in Anion Binding CalixarenesQureshi, N., Yufit, D., Steed, K., Howard, J., & Steed, J. (2014). Hydrogen Bonding Effects in Anion Binding Calixarenes. CrystEngComm, 16(36), 8413-8420. https://doi.org/10.1039/c4ce01240b
- Supramolecular Gel Phase Crystallization: Orthogonal Self-Assembly Under Non-equilibrium ConditionsKumar, D., & Steed, J. (2014). Supramolecular Gel Phase Crystallization: Orthogonal Self-Assembly Under Non-equilibrium Conditions. Chemical Society Reviews, 44, 2080-2088. https://doi.org/10.1039/c3cs60224a
- Structure of Organic Solids at Low Temperature and High PressureLee, R., Howard, J., Probert, M., & Steed, J. (2014). Structure of Organic Solids at Low Temperature and High Pressure. Chemical Society Reviews, 43(13), 4300-4311. https://doi.org/10.1039/c4cs00046c
- Carbamoyl Radical-Mediated Synthesis and Semipinacol Rearrangement of β-Lactam DiolsBetou, M., Male, L., Steed, J. W., & Grainger, R. S. (2014). Carbamoyl Radical-Mediated Synthesis and Semipinacol Rearrangement of β-Lactam Diols. Chemistry - A European Journal, 20(21), 6505-6517. https://doi.org/10.1002/chem.201304982
- Using Gel Morphology to Control Pore ShapeFoster, J. A., Johnson, D. W., Pipenbrock, M. M., & Steed, J. W. (2014). Using Gel Morphology to Control Pore Shape. New Journal of Chemistry, 38(3), 927-932. https://doi.org/10.1039/c3nj01295f
- N-alkyl pyrrolidone ether podands as versatile alkali metal ion chelantsPerrin, A., Myers, D., Fucke, K., Musa, O. M., & Steed, J. W. (2014). N-alkyl pyrrolidone ether podands as versatile alkali metal ion chelants. Dalton Transactions, 43(8), 3153-3161. https://doi.org/10.1039/c3dt53001a
- Supramolecular assembly in a Janus-type urea systemLloyd, G. O., & Steed, J. W. (2014). Supramolecular assembly in a Janus-type urea system. Chemical Communications, 50(12), 1426-1428. https://doi.org/10.1039/c3cc48603f
- Blending gelators to tune gel properties and probe anion-induced disassemblyFoster, J., Edkins, R., Cameron, G., Colgin, N., Fucke, K., Ridgeway, S., Crawford, A., Marder, T., Beeby, A., Cobb, S., & Steed, J. (2014). Blending gelators to tune gel properties and probe anion-induced disassembly. Chemistry - A European Journal, 20(1), 279-291. https://doi.org/10.1002/chem.201303153
- Triggered Formation of Thixotropic Hydrogels by Balancing Competitive Supramolecular SynthonsLiu, K., & Steed, J. (2013). Triggered Formation of Thixotropic Hydrogels by Balancing Competitive Supramolecular Synthons. Soft Matter, 9(48), 11699-11705. https://doi.org/10.1039/c3sm51949j
- Unexpected Low Temperature Behaviour of Piroxicam MonohydrateFucke, K., Edwards, A., Probert, M., Tallentire, S., Howard, J., & Steed, J. (2013). Unexpected Low Temperature Behaviour of Piroxicam Monohydrate. ChemPhysChem, 14(4), 675-679. https://doi.org/10.1002/cphc.201200316
- The role of co-crystals in pharmaceutical designSteed, J. (2013). The role of co-crystals in pharmaceutical design. Trends in Pharmacological Sciences, 34(3), 185-193. https://doi.org/10.1016/j.tips.2012.12.003
- First glimpse at a calixarene clathrateSteed, J. (2013). First glimpse at a calixarene clathrate. Chemical Communications, 49(2), 114-117. https://doi.org/10.1039/c2cc32607h
- Halogen-Bonding-Triggered Supramolecular Gel FormationMeazza, L., Foster, J., Fucke, K., Metrangolo, P., Resnati, G., & Steed, J. (2013). Halogen-Bonding-Triggered Supramolecular Gel Formation. Nature Chemistry, 5(1), 42-47. https://doi.org/10.1038/nchem.1496
- The Chemistry of Low Dosage Clathrate Hydrate InhibitorsPerrin, A., Musa, O., & Steed, J. (2013). The Chemistry of Low Dosage Clathrate Hydrate Inhibitors. Chemical Society Reviews, 42(5), 1996-2015. https://doi.org/10.1039/c2cs35340g
- Anion receptor coordination tripods capped by [9]ane-S3Todd, A., Swinburne, A., Goeta, A., & Steed, J. (2013). Anion receptor coordination tripods capped by [9]ane-S3. New Journal of Chemistry, 37(1), 89-96. https://doi.org/10.1039/c2nj40401j
- Overcoming the solvation shell during the crystallisation of diatrizoic acid from dimethylsulfoxideFucke, K., Howard, J., & Steed, J. (2012). Overcoming the solvation shell during the crystallisation of diatrizoic acid from dimethylsulfoxide. Chemical Communications, 48(99), 12065-12067. https://doi.org/10.1039/c2cc35995b
- New insights into an Old Molecule: Interaction Energies of Theophylline Crystal FormsFucke, K., McIntyre, G., Wilkinson, C., Henry, M., Howard, J., & Steed, J. (2012). New insights into an Old Molecule: Interaction Energies of Theophylline Crystal Forms. Crystal Growth and Design, 12(3), 1395-1401. https://doi.org/10.1021/cg201499s
- Novel Capsular Aggregates from Flexible Tripodal Triureas with Cs SymmetryAlajarin, M., Orenes, R., Howard, J. A., Spencer, E. C., Steed, J. W., & Pastor, A. (2012). Novel Capsular Aggregates from Flexible Tripodal Triureas with Cs Symmetry. Chemistry - A European Journal, 18(8), 2389-2397. https://doi.org/10.1002/chem.201102246
- A new water•••Na+ coordination motif in an unexpected diatrizoic acid disodium salt crystal formFucke, K., Peach, M., Howard, J., & Steed, J. (2012). A new water•••Na+ coordination motif in an unexpected diatrizoic acid disodium salt crystal form. Chemical Communications, 48(79), 9822-9824. https://doi.org/10.1039/c2cc32766j
- Mechanochemistry: opportunities for new and cleaner synthesisJames, S., Adams, C., Bolm, C., Braga, D., Collier, P., Friščić, T., Grepioni, F., Harris, K., Hyett, G., Jones, W., Krebs, A., Mack, J., Maini, L., Orpen, A., Parkin, I., Shearouse, W., Steed, J., & Waddell, D. (2012). Mechanochemistry: opportunities for new and cleaner synthesis. Chemical Society Reviews, 41(1), 413-447. https://doi.org/10.1039/c1cs15171a
- Two Different Hydrogen Bond Donor Ligands Together: A Selectivity Improvement in Organometallic {Re(CO)3} Anion HostsIon, L., Nieto, S., Pérez, J., Riera, L., Riera, V., Díaz, J., López, R., Anderson, K. M., & Steed, J. W. (2011). Two Different Hydrogen Bond Donor Ligands Together: A Selectivity Improvement in Organometallic {Re(CO)3} Anion Hosts. Inorganic Chemistry, 50(17), 8524-8531. https://doi.org/10.1021/ic201120s
- Anion-switchable supramolecular gels for controlling pharmaceutical crystal growthFoster, J., Piepenbrock, M., Lloyd, G., Clarke, N., Howard, J., & Steed, J. (2010). Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nature Chemistry, 2(12), 1037-1043. https://doi.org/10.1038/nchem.859
- Shear induced gelation in a copper(II) metallogel: new aspects of ion-tunable rheology and gel-reformation by external chemical stimuliPiepenbrock, M., Clarke, N., & Steed, J. (2010). Shear induced gelation in a copper(II) metallogel: new aspects of ion-tunable rheology and gel-reformation by external chemical stimuli. Soft Matter, 6(15), 3541-3547. https://doi.org/10.1039/c0sm00313a
- Designing Co-Crystals of Pharmaceutically Relevant Compounds That Crystallize with Z’ > 1Anderson, K., Probert, M., Whiteley, C., Rowland, A., Goeta, A., & Steed, J. (2009). Designing Co-Crystals of Pharmaceutically Relevant Compounds That Crystallize with Z’ > 1. Crystal Growth and Design, 9(2), 1082-1087. https://doi.org/10.1021/cg8009089
- Gelation is crucially dependent on functional group orientation and may be tuned by anion bindingPiepenbrock, M., Lloyd, G., Clarke, N., & Steed, J. (2008). Gelation is crucially dependent on functional group orientation and may be tuned by anion binding. Chemical Communications, 23, 2644-2646. https://doi.org/10.1039/b804259d
- Induced Fit Interanion Discrimination by Binding-Induced Excimer FormationFilby, M. H., Dickson, S. J., Zaccheroni, N., Prodi, L., Bonacchi, S., Montalti, M., Paterson, M., Humphries, T., Chiorboli, C., & Steed, J. (2008). Induced Fit Interanion Discrimination by Binding-Induced Excimer Formation. Journal of the American Chemical Society, 130(12), 4105-4113. https://doi.org/10.1021/ja711012d
- Structure Calculation of an Elastic Hydrogel from Sonication of Rigid Small Molecule ComponentsAnderson, K., Day, G., Paterson, M., Byrne, P., Clarke, N., & Steed, J. (2008). Structure Calculation of an Elastic Hydrogel from Sonication of Rigid Small Molecule Components. Angewandte Chemie International Edition, 47(6), 1058-1062. https://doi.org/10.1002/anie.200703785
- A modular approach to organic, coordination complex and polymer based podand hosts for anions.Filby, M., & Steed, J. (2006). A modular approach to organic, coordination complex and polymer based podand hosts for anions. Coordination Chemistry Reviews, 250(23-24), 3200-3218. https://doi.org/10.1016/j.ccr.2006.06.004
- On the protonation of a macrobicyclic cage: an inert tribenzylamine fragment and three robust aminophosphonium units.Alajarín, M., López-Leonardo, C., Berná, J., & Steed, J. (2006). On the protonation of a macrobicyclic cage: an inert tribenzylamine fragment and three robust aminophosphonium units. Tetrahedron Letters, 47(30), 5405-5408. https://doi.org/10.1016/j.tetlet.2006.05.112
- Crystal engineering with ethynylbenzenes Part 2. Structures of 4-trimethylsilylethynyl-N,N-dimethylaniline, and 4-ethynyl-N,N-dimethylaniline with Z '=12 and a single-crystal to single-crystal phase transition at 122.5 +/- 2 KBatsanov, A., Collings, J., Ward, R., Goeta, A., Porres, L., Beeby, A., Howard, J., Steed, J., & Marder, T. (2006). Crystal engineering with ethynylbenzenes Part 2. Structures of 4-trimethylsilylethynyl-N,N-dimethylaniline, and 4-ethynyl-N,N-dimethylaniline with Z ’=12 and a single-crystal to single-crystal phase transition at 122.5 +/- 2 K. CrystEngComm, 8, 622-628. https://doi.org/10.1039/b606327f
- Helical or polar guest-dependent Z‘ = 1.5 or Z‘ = 2 forms of a sterically hindered bis(urea) clathrate.Todd, A., Anderson, K., Byrne, P., Goeta, A., & Steed, J. (2006). Helical or polar guest-dependent Z‘ = 1.5 or Z‘ = 2 forms of a sterically hindered bis(urea) clathrate. Crystal Growth and Design, 6(8), 1750-1752. https://doi.org/10.1021/cg060318o
- A conformationally flexible, urea-based tripodal anion receptor: Solid-state, solution, and theoretical studiesTurner, D., Paterson, M., & Steed, J. (2006). A conformationally flexible, urea-based tripodal anion receptor: Solid-state, solution, and theoretical studies. Journal of Organic Chemistry, 71(4), 1598-1608. https://doi.org/10.1021/jo052339f
- Synthesis, characterisation and natural abundance Os-187 NMR spectroscopy of hydride bridged triosmium clusters with chiral diphosphine ligandsStchedroff, M., Moberg, V., Rodriguez, E., Aliev, A., Böttcher, J., Steed, J., Nordlander, E., Monari, M., & Deeming, A. (2006). Synthesis, characterisation and natural abundance Os-187 NMR spectroscopy of hydride bridged triosmium clusters with chiral diphosphine ligands. Inorganica Chimica Acta, 359(3), 926-937. https://doi.org/10.1016/j.ica.2005.06.048
- Anion-binding mode in a sulfanylphenyl urea complex : solid state symmetry breaking and solution chelation.Russell, J. M., Parker, A. D. M., Radosavljevic-Evans, I., Howard, J. A. K., & Steed, J. W. (2006). Anion-binding mode in a sulfanylphenyl urea complex : solid state symmetry breaking and solution chelation. CrystEngComm, 8(2), 119-122. https://doi.org/10.1039/b516962c
- When Z '=2 is better than Z '=1-supramolecular centrosymmetric hydrogen-bonded dimers in chiral systemsAnderson, K., Afarinkia, K., Yu, H., Goeta, A., & Steed, J. (2006). When Z ’=2 is better than Z ’=1-supramolecular centrosymmetric hydrogen-bonded dimers in chiral systems. Crystal Growth and Design, 6(9), 2109-2113. https://doi.org/10.1021/cg0603265
- Anion binding inhibition of the formation of a helical organogelStanley, C., Clarke, N., Anderson, K., Elder, J., Lenthall, J., & Steed, J. (2006). Anion binding inhibition of the formation of a helical organogel. Chemical Communications, 30, 3199-3201. https://doi.org/10.1039/b606373j
- Modular assembly of a preorganised, ditopic receptor for dicarboxylates.Filby, M., Humphries, T., Turner, D., Kataky, R., Kruusma, J., & Steed, J. (2006). Modular assembly of a preorganised, ditopic receptor for dicarboxylates. Chemical Communications, 2006(2), 156-158. https://doi.org/10.1039/b512779c
- A modular approach to anion binding podands: adaptability in design and synthesis leads to adaptability in propertiesSteed, J. (2006). A modular approach to anion binding podands: adaptability in design and synthesis leads to adaptability in properties. Chemical Communications, 2006(25), 2637-2649. https://doi.org/10.1039/b601511e
- Simultaneous anion and cation binding by a simple polymer-bound ureidopyridyl ligand.Russell, J. M., Parker, A. D. M., Radosavljevie-Evans, I., Howard, J. A. K., & Steed, J. W. (2006). Simultaneous anion and cation binding by a simple polymer-bound ureidopyridyl ligand. Chemical Communications, 2006(3), 269-271. https://doi.org/10.1039/b513820e
- Pyridinium CH center dot center dot center dot anion and pi-stacking interactions in modular tripodal anion binding hosts: ATP binding and solid-state chiral inductionBelcher, W., Fabre, M., Farhan, T., & Steed, J. (2006). Pyridinium CH center dot center dot center dot anion and pi-stacking interactions in modular tripodal anion binding hosts: ATP binding and solid-state chiral induction. Organic and Biomolecular Chemistry, 4(5), 781-786. https://doi.org/10.1039/b516027h
- Unusual variations in the incidence of Z ' > 1 in oxo-anion structuresAnderson, K., Goeta, A., Hancock, K., & Steed, J. (2006). Unusual variations in the incidence of Z ’ > 1 in oxo-anion structures. Chemical Communications, 2006(20), 2138-2140. https://doi.org/10.1039/b602492k
- Complex Formation and Rearrangement Reactions of the Phosphine Hydride Anions [OsH3(PPh3)3]- and [IrH2(PPh3)3]-Guilera, G., McGrady, G., Steed, J., & Jones, A. (2006). Complex Formation and Rearrangement Reactions of the Phosphine Hydride Anions [OsH3(PPh3)3]- and [IrH2(PPh3)3]-. Organometallics, 25(1), 122-127. https://doi.org/10.1021/om050391w
- Cooperative hydrogen-bonding effects in a water square: A single-crystal neutron and partial atomic charges and hardness analysis studyTurner, D., Henry, M., Wilkinson, C., McIntyre, G., Mason, S., Goeta, A., & Steed, J. (2005). Cooperative hydrogen-bonding effects in a water square: A single-crystal neutron and partial atomic charges and hardness analysis study. Journal of the American Chemical Society, 127(31), 11063-11074. https://doi.org/10.1021/ja052081a
- A study of Co-2(alkyne)(binap)(CO)(4) complexes (BINAP = (1,1 '-binaphthalene)-2,2 '-diylbis(diphenylphosphine))Gibson, S., Kaufmann, K., Loch, J., Steed, J., & White, A. (2005). A study of Co-2(alkyne)(binap)(CO)(4) complexes (BINAP = (1,1 ’-binaphthalene)-2,2 ’-diylbis(diphenylphosphine)). Chemistry - A European Journal, 11(8), 2566-2576.
- Alkene-arene meta photocycloadditions with a four-carbon-atom tether: Efficient approach toward the polycyclic ring systems of aphidicolin and stemodinoneBoyd, J., Greaves, N., Kettle, J., Russell, A., & Steed, J. (2005). Alkene-arene meta photocycloadditions with a four-carbon-atom tether: Efficient approach toward the polycyclic ring systems of aphidicolin and stemodinone. Angewandte Chemie International Edition, 44(6), 944-946.
- Hydrogen bonds between ammonium ions and aromatic rings exist and have key consequences on solid-state and solution phase propertiesIlioudis, C., Bearpark, M., & Steed, J. (2005). Hydrogen bonds between ammonium ions and aromatic rings exist and have key consequences on solid-state and solution phase properties. New Journal of Chemistry, 29(1), 64-67.
- Stereoselective synthesis of 2,5-disubstituted-1,4-oxathiane S-oxidesBedford, S., Grainger, R., Steed, J., & Tisselli, P. (2005). Stereoselective synthesis of 2,5-disubstituted-1,4-oxathiane S-oxides. Organic and Biomolecular Chemistry, 3(3), 404-406.
- Anion binding by Ag(I) complexes of urea-substituted pyridyl ligandsTurner, D., Smith, B., Spencer, E., Goeta, A., Evans, I., Tocher, D., Howard, J., & Steed, J. (2005). Anion binding by Ag(I) complexes of urea-substituted pyridyl ligands. New Journal of Chemistry, 29(1), 90-98.
- Polyaza metacyclophanes as ditopic anion receptorsIlioudis, C., & Steed, J. (2005). Polyaza metacyclophanes as ditopic anion receptors. Organic and Biomolecular Chemistry, 3(16), 2935-2945.
- Bis(carbene)pyridine complexes of the early to middle transition metals: Survey of ethylene oligomerization and polymerization capabilityMcGuinness, D., Gibson, V., & Steed, J. (2004). Bis(carbene)pyridine complexes of the early to middle transition metals: Survey of ethylene oligomerization and polymerization capability. Organometallics, 23(26), 6288-6292.
- The R-1(2)(6) hydrogen-bonded synthon in neutral urea and metal-bound halide systemsTurner, D., Smith, B., Goeta, A., Evans, I., Tocher, D., Howard, J., & Steed, J. (2004). The R-1(2)(6) hydrogen-bonded synthon in neutral urea and metal-bound halide systems. CrystEngComm, 6, 633-641.
- A highly efficient, preorganized macrobicyclic receptor for halides based on CH··· and NH···anion interactionsIlioudis, C., Tocher, D., & Steed, J. (2004). A highly efficient, preorganized macrobicyclic receptor for halides based on CH··· and NH···anion interactions. Journal of the American Chemical Society, 126(39), 12395-12402. https://doi.org/10.1021/ja047070g
- Synthesis of meta- and paracyclophanes containing unsaturated amino acid residuesGibson, S., Jones, J., Kalindjian, S., Knight, J., Mainolfi, N., Rudd, M., Steed, J., Tozer, M., & Wright, P. (2004). Synthesis of meta- and paracyclophanes containing unsaturated amino acid residues. Tetrahedron, 60(32), 6945-6958.
- Complexation of I-4(2-) and I-8(2-) by protonated azacyclophanesIlioudis, C., & Steed, J. (2004). Complexation of I-4(2-) and I-8(2-) by protonated azacyclophanes. CrystEngComm, 6, 239-242.
- Expanding metallaborane chemistry: an octahedral BH6 moiety supported through M-H-B bridgesMcGrady, G., Guilera, G., Steed, J., & Kaltsoyannis, N. (2004). Expanding metallaborane chemistry: an octahedral BH6 moiety supported through M-H-B bridges. New Journal of Chemistry, 28(4), 444-446.
- Modular approach to anion binding and sensingSteed, J. (2004). Modular approach to anion binding and sensing. Abstracts of Papers: American Chemical Society Meetings, 227, U1234-U1234.
- Linear distortion of octahedral metal centres by multiple hydrogen bonds in modular ML4 systemsTurner, D., Hursthouse, M., Light, A., & Steed, J. (2004). Linear distortion of octahedral metal centres by multiple hydrogen bonds in modular ML4 systems. Chemical Communications, 2004(12), 1354-1355. https://doi.org/10.1039/B402884H
- A modular, self-assembled, separated ion pair binding systemTurner, D., Spencer, E., Howard, J., Tocher, D., & Steed, J. (2004). A modular, self-assembled, separated ion pair binding system. Chemical Communications, 12, 1352-1353. https://doi.org/10.1039/B402882A
- The unusual solid state structure of heroin hydrochloride monohydrate and its selective detection using NQR spectroscopyBalchin, E., Malcolme-Lawes, D., Rowe, M., Smith, J., Bearpark, M., Steed, J., Wu, W., Horsewill, A., & Stephenson, D. (2004). The unusual solid state structure of heroin hydrochloride monohydrate and its selective detection using NQR spectroscopy. New Journal of Chemistry, 28(11), 1309-1314.
- Molecular containers: Design approaches and applicationsTurner, D., Pastor, A., Alajarin, M., & Steed, J. (2004). Molecular containers: Design approaches and applications. Supramolecular Assembly Via Hydrogen Bonds I, 108, 97-168.
- Slow anion exchange, conformational equilibria, and fluorescent sensing in venus flytrap aminopyridinium-based anion hostsWallace, K., Belcher, W., Turner, D., Syed, K., & Steed, J. (2003). Slow anion exchange, conformational equilibria, and fluorescent sensing in venus flytrap aminopyridinium-based anion hosts. Journal of the American Chemical Society, 125(32), 9699-9715. https://doi.org/10.1021/ja034921w
- Should solid-state molecular packing have to obey the rules of crystallographic symmetry?Steed, J. (2003). Should solid-state molecular packing have to obey the rules of crystallographic symmetry?. CrystEngComm, 5(32), 169-179. https://doi.org/10.1039/b304631a
- Influence of hydrogen bonding on coordination polymer assemblyApplegarth, L., Goeta, A., & Steed, J. (n.d.). Influence of hydrogen bonding on coordination polymer assembly. Chemical Communications, 18, 2405-2406.
- Anion and cation binding by a pendant arm cyclam and its macrobicyclic derivativesChanna, A., & Steed, J. (n.d.). Anion and cation binding by a pendant arm cyclam and its macrobicyclic derivatives. Dalton Transactions, 14, 2455-2461.
- Multiple nitrene insertions into metal-sulfur bonds of dithiocarbamate complexes: synthesis of sulfido-amido and zwitterionic tetraamido complexesHogarth, G., Holman, K., Pateman, A., Sella, A., Steed, J., & Richards, I. (n.d.). Multiple nitrene insertions into metal-sulfur bonds of dithiocarbamate complexes: synthesis of sulfido-amido and zwitterionic tetraamido complexes. Dalton Transactions, 16, 2688-2695.
- Modular nanometer-scale structuring of gel fibres by sequential self-organizationApplegarth, L., Clark, N., Richardson, A. C., Parker, A. D. M., Radosavljevic-Evans, I., Goeta, A. E., Howard, J. A. K., & Steed, J. W. (n.d.). Modular nanometer-scale structuring of gel fibres by sequential self-organization. Chemical Communications, 43, 5423-5425.

