Staff profile

| Affiliation |
|---|
| Assistant Professor in the Department of Chemistry |
Biography
Research Interests
We are principally interested in the fundamental synthetic, chemical and physical properties of luminescent compounds. They are characterised by spectroscopic (NMR, IR, UV-vis, MS, Raman), computational (DFT) and diffraction (X-ray) methods available from the excellent departmental research facilities here along with (spectro)electrochemical and photophysical measurements to explore their emission properties. Luminescent compounds of interest include ON/OFF photoswitches, phosphorescent metal complexes and thermally activated delayed fluorescence (TADF) molecules.
Prompt fluorescence molecules
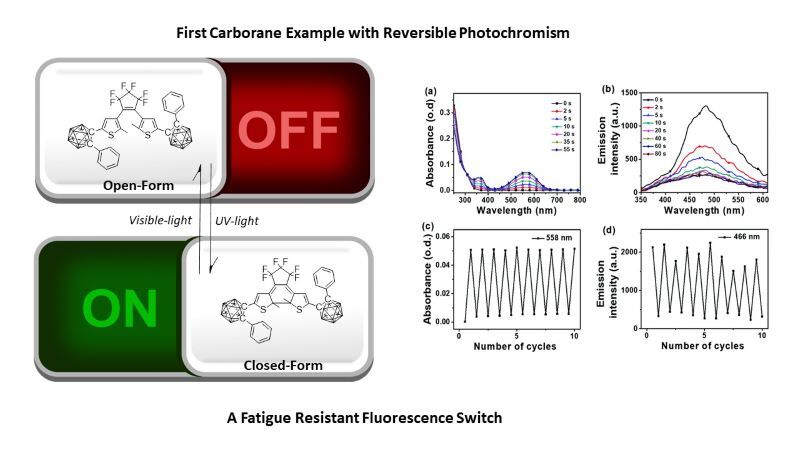
Compounds containing carboranes (stable clusters of boron, carbon and hydrogen) have intriguing photophysical properties. Some derivatives of ortho-carborane (1,2-C2B10H12) have dual emissions where one emission is due to charge transfer (CT) or excimer/aggregation formation.
Chem. Commun., 2021, 57, 9466-9469 https://doi.org/10.1039/D1CC03248H
Chem. Sci., 2022, 13, 5205-5219 https://doi.org/10.1039/D1SC06867A
Nat. Commun., 2024, 15, 5205-5219 https://doi.org/10.1038/s41467-024-47384-4
Phosphorescent complexes
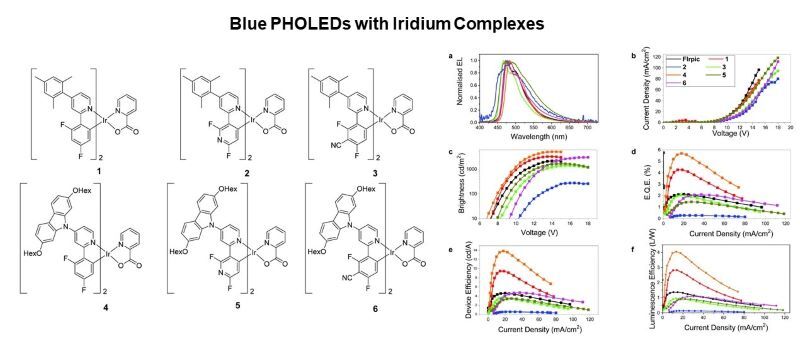
Certain metal complexes are used as emitters in phosphorescent organic light-emitting diodes (PHOLEDs). Iridium complexes are shown to emit desirable blue colours and can be incorporated in PHOLEDs with high efficiencies.
Dalton Trans., 2020, 49, 2190-2208 https://doi.org/10.1039/C9DT04672K
Organometallics, 2022, 41, 2487-2493 https://doi.org/10.1021/acs.organomet.2c00292
Inorg. Chem., 2023, 62, 2793-2805 https://doi.org/10.1021/acs.inorgchem.2c03934
Dalton Trans., 2024, 53, 17518-17524 https://doi.org/10.1039/D4DT02321H
Thermally activated delayed fluorescence (TADF)
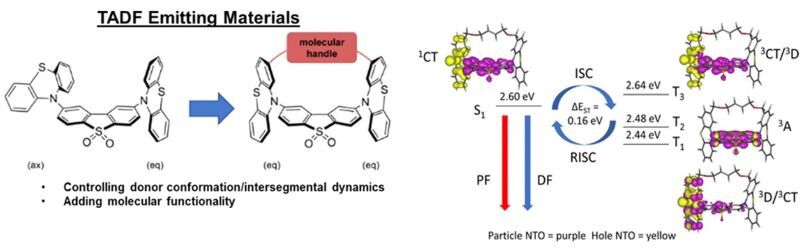
TADF is a recent area of intense research as inexpensive organic compounds are exploited as suitable emitters in organic light-emitting diodes (OLEDs) due to high efficiencies made possible by harvesting energy from both singlet and triplet states like in expensive iridium complexes.
Mater. Chem. Front., 2020, 4, 3602-3615 https://doi.org/10.1039/D0QM00429D
Chem. Mater., 2021, 33, 3066–3080 https://doi.org/10.1021/acs.chemmater.0c03783
J. Org. Chem., 2021, 86, 429–445 https://doi.org/10.1021/acs.joc.0c02174
Chem. Mater., 2024, 36, 7135–7150 https://doi.org/10.1021/acs.chemmater.4c00850
Research interests
- Computational DFT
- Luminescence
Publications
Chapter in book
- Polyhedral CarboranesFox, M. (2007). Polyhedral Carboranes. In D. M. P. Mingos & R. H. Crabtree (Eds.), Comprehensive Organometallic Chemistry III. (pp. 49-112). Elsevier. https://doi.org/10.1016/B0-08-045047-4/00043-1
- Towards experimental mapping of the mechanism of heteroborane isomerisationBarbera, G., Dunn, S., Fox, M., Garrioch, R., Hodson, B., Low, K., Rosair, G., Teixidor, F., Vinas, C., Welch, A. J., & Weller, A. (2000). Towards experimental mapping of the mechanism of heteroborane isomerisation. In M. G. Davidson, K. Wade, T. Marder, & A. K. Hughes (Eds.), Contemporary Boron Chemistry (pp. 329-336).
- Studies of icosahedral carboranes with iminotris(dimethylamino)phosphorane, HNP(NMe2)(3)Davidson, M., Fox, M., Gray, F., Hibbert, T., & Wade, K. (2000). Studies of icosahedral carboranes with iminotris(dimethylamino)phosphorane, HNP(NMe2)(3). In M. Davidson, A. Hughes, T. Marder, & K. Wade (Eds.), Contemporary Boron Chemistry (pp. 223-228). Royal Society of Chemistry.
- Synthesis and structural characterisation of the anion nido-[B8H11](-), and new insights into the structures of other octaborane speciesCondick, P., Fox, M., Greatrex, R., & Ormsby, D. (2000). Synthesis and structural characterisation of the anion nido-[B8H11](-), and new insights into the structures of other octaborane species. In M. G. Davidson, K. Wade, T. Marder, & A. K. Hughes (Eds.), Contemporary Boron Chemistry (pp. 179-186). Royal Society of Chemistry.
- Reactions of unsaturated hydrocarbons with small boranes: New insights and recent advancesGreatrex, R., & Fox, M. A. (1998). Reactions of unsaturated hydrocarbons with small boranes: New insights and recent advances. In J. Casanova (Ed.), Borane, Carborane, Carbocation Continuum (pp. 289-305).
- The borane-carborane structural pattern: Some correlations and implicationsGreatrex, R., & Fox, M. (1998). The borane-carborane structural pattern: Some correlations and implications. In J. Casanova (Ed.), The Borane, Carborane, Carbocation Continuum (pp. 57-84).
Journal Article
- Diiridium(III) Complexes with Fluorenylpyridyl Cyclometalating and μ 2 ‐Oxamidato Bridging Ligands and their High Efficiency Phosphorescent Solution‐Processed OLEDsM’hamedi, A., Fox, M. A., Batsanov, A. S., Al‐Attar, H. A., & Bryce, M. R. (2025). Diiridium(III) Complexes with Fluorenylpyridyl Cyclometalating and μ 2 ‐Oxamidato Bridging Ligands and their High Efficiency Phosphorescent Solution‐Processed OLEDs. European Journal of Inorganic Chemistry, 28(6), Article e202400745. https://doi.org/10.1002/ejic.202400745
- Dual phosphorescent emissions from conformers of iridium complex rotors.Hsu, Y., Bhagani, C., Aguilar, J. A., Fox, M. A., Yufit, D., Davidson, R., & Beeby, A. (2024). Dual phosphorescent emissions from conformers of iridium complex rotors. Dalton Transactions, 53(43), 17518-17524. https://doi.org/10.1039/D4DT02321H
- Exciplex, Not Heavy-Atom Effect, Controls the Triplet Dynamics of a Series of Sulfur-Containing Thermally Activated Delayed Fluorescence MoleculesÖner, S., Kuila, S., Stavrou, K., Danos, A., Fox, M. A., Monkman, A. P., & Bryce, M. R. (2024). Exciplex, Not Heavy-Atom Effect, Controls the Triplet Dynamics of a Series of Sulfur-Containing Thermally Activated Delayed Fluorescence Molecules. Chemistry of Materials, 36(15), 7135-7150. https://doi.org/10.1021/acs.chemmater.4c00850
- Optically induced charge-transfer in donor-acceptor-substituted p - and m - C₂ B₁₀ H₁₂ carboranesWu, L., Holzapfel, M., Schmiedel, A., Peng, F., Moos, M., Mentzel, P., Shi, J., Neubert, T., Bertermann, R., Finze, M., Fox, M. A., Lambert, C., & Ji, L. (2024). Optically induced charge-transfer in donor-acceptor-substituted p - and m - C₂ B₁₀ H₁₂ carboranes. Nature Communications, 15(1), Article 3005. https://doi.org/10.1038/s41467-024-47384-4
- Structural Diversity in Cyclometalated Diiridium(III) Complexes with Bridging syn and anti μ2‐Oxamidato and μ2‐Dithioxamidato LigandsM’hamedi, A., Batsanov, A. S., Fox, M. A., Aguilar, J. A., & Bryce, M. R. (2023). Structural Diversity in Cyclometalated Diiridium(III) Complexes with Bridging syn and anti μ2‐Oxamidato and μ2‐Dithioxamidato Ligands. European Journal of Inorganic Chemistry, 26(34), Article e202300423. https://doi.org/10.1002/ejic.202300423
- Tuning Emission Lifetimes of Ir(C^N)2(acac) Complexes with Oligo(phenyleneethynylene) GroupsDavidson, R., Hsu, Y., Fox, M. A., Aguilar, J. A., Yufit, D., & Beeby, A. (2023). Tuning Emission Lifetimes of Ir(C^N)2(acac) Complexes with Oligo(phenyleneethynylene) Groups. Inorganic Chemistry, 62(6), 2793-2805. https://doi.org/10.1021/acs.inorgchem.2c03934
- Vapor Sorption and Halogen-Bond-Induced Solid-Form Rearrangement of a Porous PharmaceuticalAndrews, J. L., Yufit, D. S., McCabe, J. F., Fox, M. A., & Steed, J. W. (2023). Vapor Sorption and Halogen-Bond-Induced Solid-Form Rearrangement of a Porous Pharmaceutical. Crystal Growth & Design, 23(4), 2628-2633. https://doi.org/10.1021/acs.cgd.2c01464
- Iridium‐Catalysed C−H Borylation of Fluoroarenes: Insights into the Balance between Steric and Electronic Control of RegioselectivityDing, M., Reuven, J. A., Hones, A. C., Fox, M. A., & Steel, P. G. (2022). Iridium‐Catalysed C−H Borylation of Fluoroarenes: Insights into the Balance between Steric and Electronic Control of Regioselectivity. European Journal of Organic Chemistry, 2022(47). https://doi.org/10.1002/ejoc.202201005
- Divergent Approach for Tris-Heteroleptic Cyclometalated Iridium Complexes Using Triisopropylsilylethynyl-Substituted SynthonsEdkins, R. M., Hsu, Y., Fox, M. A., Yufit, D., Beeby, A., & Davidson, R. J. (2022). Divergent Approach for Tris-Heteroleptic Cyclometalated Iridium Complexes Using Triisopropylsilylethynyl-Substituted Synthons. Organometallics, 41(17), 2487-2493. https://doi.org/10.1021/acs.organomet.2c00292
- Structure and hydration of polyvinylpyrrolidone-hydrogen peroxideChambers, L. I., Yufit, D. S., Fox, M. A., Musa, O. M., & Steed, J. W. (2022). Structure and hydration of polyvinylpyrrolidone-hydrogen peroxide. Chemical Communications, 58(1), 80-83. https://doi.org/10.1039/d1cc06047c
- Thermodynamic equilibrium between locally excited and charge-transfer states through thermally activated charge transfer in 1-(pyren-2′-yl)-o-carboraneJi, L., Riese, S., Schmiedel, A., Holzapfel, M., Fest, M., Nitsch, J., Curchod, B. F., Friedrich, A., Wu, L., Al Mamari, H. H., Hammer, S., Pflaum, J., Fox, M. A., Tozer, D. J., Finze, M., Lambert, C., & Marder, T. B. (2022). Thermodynamic equilibrium between locally excited and charge-transfer states through thermally activated charge transfer in 1-(pyren-2′-yl)-o-carborane. Chemical Science, 13, 5205-5219. https://doi.org/10.1039/d1sc06867a
- Carborane photochromism: A fatigue resistant carborane switchLi, C., Aldred, M. P., Harder, R. A., Chen, Y., Yufit, D. S., Zhu, M., & Fox, M. A. (2021). Carborane photochromism: A fatigue resistant carborane switch. Chemical Communications, 57(74), 9466-9469. https://doi.org/10.1039/d1cc03248h
- Vibrational Damping Reveals Vibronic Coupling in Thermally Activated Delayed Fluorescence MaterialsHempe, M., Kukhta, N. A., Danos, A., Fox, M. A., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2021). Vibrational Damping Reveals Vibronic Coupling in Thermally Activated Delayed Fluorescence Materials. Chemistry of Materials, 33(9), 3066-3080. https://doi.org/10.1021/acs.chemmater.0c03783
- Nonlinear optical properties of meso-Tetra(fluorenyl)porphyrins peripherally functionalized with one to four ruthenium alkynyl substituentsZhang, X., Shi, L., Fox, M. A., Barlow, A., Morshedi, M., Cifuentes, M. P., Humphrey, M. G., Mongin, O., Paul, F., & Paul-Roth, C. O. (2021). Nonlinear optical properties of meso-Tetra(fluorenyl)porphyrins peripherally functionalized with one to four ruthenium alkynyl substituents. Dyes and Pigments, 188, Article 109155. https://doi.org/10.1016/j.dyepig.2021.109155
- Cyclophane Molecules Exhibiting Thermally Activated Delayed Fluorescence: Linking Donor Units to Influence Molecular ConformationHempe, M., Harrison, A. K., Ward, J. S., Batsanov, A. S., Fox, M. A., Dias, F. B., & Bryce, M. R. (2021). Cyclophane Molecules Exhibiting Thermally Activated Delayed Fluorescence: Linking Donor Units to Influence Molecular Conformation. Journal of Organic Chemistry, 86(1), 429-445. https://doi.org/10.1021/acs.joc.0c02174
- HFO-1234yf as a CF3-building block: Synthesis and Chemistry of CF3-ynonesMurray, B. J., Marsh, T. G., Yufit, D. S., Fox, M. A., Harsanyi, A., Boulton, L. T., & Sandford, G. (2020). HFO-1234yf as a CF3-building block: Synthesis and Chemistry of CF3-ynones. European Journal of Organic Chemistry, 2020(39), 6236-6244. https://doi.org/10.1002/ejoc.202001071
- Unusual dual-emissive heteroleptic iridium complexes incorporating TADF cyclometalating ligandsBenjamin, H., Zheng, Y., Kozhevnikov, V. N., Siddle, J. S., O’Driscoll, L. J., Fox, M. A., Batsanov, A. S., Griffiths, G. C., Dias, F. B., Monkman, A. P., & Bryce, M. R. (2020). Unusual dual-emissive heteroleptic iridium complexes incorporating TADF cyclometalating ligands. Dalton Transactions, 49(7), 2190-2208. https://doi.org/10.1039/c9dt04672k
- Exploiting trifluoromethyl substituents for tuning orbital character of singlet and triplet states to increase the rate of thermally activated delayed fluorescenceWard, J. S., Danos, A., Stachelek, P., Fox, M. A., Batsanov, A. S., Monkman, A. P., & Bryce, M. R. (2020). Exploiting trifluoromethyl substituents for tuning orbital character of singlet and triplet states to increase the rate of thermally activated delayed fluorescence. Materials Chemistry Frontiers, 4(12). https://doi.org/10.1039/d0qm00429d
- Metallacarborane Assemblies as Effective Antimicrobial Agents, Including a Highly Potent Anti-MRSA AgentRomero, I., Martinez-Medina, M., Camprubí-Font, C., Bennour, I., Moreno, D., Martínez-Martínez, L., Teixidor, F., Fox, M. A., & Viñas, C. (2020). Metallacarborane Assemblies as Effective Antimicrobial Agents, Including a Highly Potent Anti-MRSA Agent. Organometallics, 39(23), 4253-4264. https://doi.org/10.1021/acs.organomet.0c00315
- Fluorenylporphyrins functionalized by electrochromic ruthenium units as redox-triggered fluorescence switchesZhang, X., Abid, S., Shi, L., Williams, J. G., Fox, M. A., Miomandre, F., Tourbillon, C., Audibert, J., Mongin, O., Paul, F., & Paul-Roth, C. O. (2019). Fluorenylporphyrins functionalized by electrochromic ruthenium units as redox-triggered fluorescence switches. Dalton Transactions, 48(31), 11897-11911. https://doi.org/10.1039/c9dt02087j
- Unravelling the Complexities of Pseudocontact Shift Analysis in Lanthanide Coordination Complexes of Differing SymmetryParker, D., Suturina, E., Harnden, A., Batsanov, A., Fox, M., Mason, K., Vonci, M., McInnes, E., & Chilton, N. (2019). Unravelling the Complexities of Pseudocontact Shift Analysis in Lanthanide Coordination Complexes of Differing Symmetry. Angewandte Chemie, 131(30), 10936-10400. https://doi.org/10.1002/anie.201906031
- Impact of Methoxy Substituents on Thermally Activated Delayed Fluorescence and Room-Temperature Phosphorescence in All-Organic Donor–Acceptor SystemsWard, J. S., Nobuyasu, R. S., Fox, M. A., Aguilar, J. A., Hall, D., Batsanov, A. S., Ren, Z., Dias, F. B., & Bryce, M. R. (2019). Impact of Methoxy Substituents on Thermally Activated Delayed Fluorescence and Room-Temperature Phosphorescence in All-Organic Donor–Acceptor Systems. Journal of Organic Chemistry, 84(7), 3801-3816. https://doi.org/10.1021/acs.joc.8b02848
- Synthetic, Structural, and Computational Studies on Heavier Tetragen and Chalcogen Triazenide ComplexesFlanagan, K. R., Parish, J. D., Fox, M. A., & Johnson, A. L. (2019). Synthetic, Structural, and Computational Studies on Heavier Tetragen and Chalcogen Triazenide Complexes. Inorganic Chemistry, 58(24), 16660-16666. https://doi.org/10.1021/acs.inorgchem.9b02757
- Bond Rotations and Heteroatom Effects in Donor-Acceptor-Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature PhosphorescenceWard, J. S., Nobuyasu, R. S., Fox, M. A., Batsanov, A. S., Santos, J., Dias, F. B., & Bryce, M. R. (2018). Bond Rotations and Heteroatom Effects in Donor-Acceptor-Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature Phosphorescence. Journal of Organic Chemistry, 83(23), 14431-14442. https://doi.org/10.1021/acs.joc.8b02187
- Selective signalling of glyphosate in water using europium luminescenceJennings, L. B., Shuvaev, S., Fox, M. A., Pal, R., & Parker, D. (2018). Selective signalling of glyphosate in water using europium luminescence. Dalton Transactions, 47(45), 16145-16154. https://doi.org/10.1039/c8dt03823f
- Monitoring the ADP/ATP ratio via induced circularly‐polarised europium luminescenceParker, D., Shuvaev, S., & Fox, M. A. (2018). Monitoring the ADP/ATP ratio via induced circularly‐polarised europium luminescence. Angewandte Chemie International Edition, 57(25), 7488-7492. https://doi.org/10.1002/anie.201801248
- Enhanced selectivity for Mg2+ with a phosphinate-based chelate: APDAP versus APTRAWalter, E. R., Fox, M. A., Parker, D., & Williams, J. G. (2018). Enhanced selectivity for Mg2+ with a phosphinate-based chelate: APDAP versus APTRA. Dalton Transactions, 47(6), 1755-1763. https://doi.org/10.1039/c7dt04698g
- Role of the Diphosphine Chelate in Emissive, Charge-Neutral Iridium(III) ComplexesLiao, J., Devereux, L. R., Fox, M. A., Yang, C., Chiang, Y., Chang, C., Lee, G., & Chi, Y. (2018). Role of the Diphosphine Chelate in Emissive, Charge-Neutral Iridium(III) Complexes. Chemistry - A European Journal, 24(3), 624-635. https://doi.org/10.1002/chem.201703482
- Bis-tridentate Ir(III) Metal Phosphors for Efficient Deep-Blue Organic Light-Emitting DiodesKuo, H., Chen, Y., Devereux, L. R., Wu, C., Fox, M. A., Kuei, C., Chi, Y., & Lee, G. (2017). Bis-tridentate Ir(III) Metal Phosphors for Efficient Deep-Blue Organic Light-Emitting Diodes. Advanced Materials, 29(33), Article 1702464. https://doi.org/10.1002/adma.201702464
- Pyridylpyrazole N^N Ligands Combined with Sulfonyl-Functionalised Cyclometalating Ligands for Blue-Emitting Iridium(III) Complexes and Solution-Processable PhOLEDsBenjamin, H., Fox, M. A., Batsanov, A. S., Al-Attar, H. A., Li, C., Ren, Z., Monkman, A. P., & Bryce, M. R. (2017). Pyridylpyrazole N^N Ligands Combined with Sulfonyl-Functionalised Cyclometalating Ligands for Blue-Emitting Iridium(III) Complexes and Solution-Processable PhOLEDs. Dalton Transactions, 46, 10996-11007. https://doi.org/10.1039/c7dt02289a
- The Contributions of Molecular Vibrations and Higher Triplet Levels to the Intersystem Crossing Mechanism in Metal-Free Organic EmittersHuang, R., Avó, J., Northey, T., Chaning-Pearce, E., dos Santos, P. L., Ward, J. S., Data, P., Etherington, M. K., Fox, M. A., Penfold, T. J., Berberan-Santos, M. N., Lima, J. C., Bryce, M. R., & Dias, F. B. (2017). The Contributions of Molecular Vibrations and Higher Triplet Levels to the Intersystem Crossing Mechanism in Metal-Free Organic Emitters. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 5(25), 6269-6280. https://doi.org/10.1039/c7tc01958k
- Geometries of 11-vertex carborane monoanion radicals with 2n+3 skeletal electron countsPatel, N., Oliva-Enrich, J., & Fox, M. (2017). Geometries of 11-vertex carborane monoanion radicals with 2n+3 skeletal electron counts. European Journal of Inorganic Chemistry, 2017(38-39), 4568-4574. https://doi.org/10.1002/ejic.201700419
- New Blatter-type radicals from a bench-stable carbeneGrant, J. A., Lu, Z., Tucker, D. E., Hockin, B. M., Yufit, D. S., Fox, M. A., Kataky, R., Chechik, V., & O’Donoghue, A. C. (2017). New Blatter-type radicals from a bench-stable carbene. Nature Communications, 8, Article 15088. https://doi.org/10.1038/ncomms15088
- Platinum(II) complexes of some unsymmetrical diphosphenesDillon, K., Fox, M., Gibson, V., Goodwin, H., & Sequiera, L. (2017). Platinum(II) complexes of some unsymmetrical diphosphenes. Journal of Organometallic Chemistry, 830, 113-119. https://doi.org/10.1016/j.jorganchem.2016.10.005
- Luminescent Pt(II) complexes featuring imidazolylidene–pyridylidene and dianionic bipyrazolate: from fundamentals to OLED fabricationsTseng, C., Fox, M., Liao, J., Ku, C., Sie, Z., Chang, C., Wang, J., Chen, Z., Lee, G., & Chi, Y. (2017). Luminescent Pt(II) complexes featuring imidazolylidene–pyridylidene and dianionic bipyrazolate: from fundamentals to OLED fabrications. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 5(6), 1420-1435. https://doi.org/10.1039/c6tc05154e
- Bright Green PhOLEDs Using Cyclometalated Diiridium(III) Complexes with Bridging Oxamidato Ligands as Phosphorescent DopantsM’hamedi, A., Fox, M. A., Batsanov, A. S., Al-Attar, H. A., Monkman, A. P., & Bryce, M. R. (2017). Bright Green PhOLEDs Using Cyclometalated Diiridium(III) Complexes with Bridging Oxamidato Ligands as Phosphorescent Dopants. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 5(27), 6777-6789. https://doi.org/10.1039/c7tc00628d
- The Role of Local Triplet Excited States and D-A Relative Orientation in Thermally Activated Delayed Fluorescence: Photophysics and DevicesDias, F., Santos, J., Graves, D., Data, P., Nobuyasu, R., Fox, M., Batsanov, A., Palmeira, T., Berberan-Santos, M., Bryce, M., & Monkman, A. (2016). The Role of Local Triplet Excited States and D-A Relative Orientation in Thermally Activated Delayed Fluorescence: Photophysics and Devices. Advanced Science, 3(12), Article 1600080. https://doi.org/10.1002/advs.201600080
- Biotransformation of fluorophenyl pyridine carboxylic acids by the model fungus Cunninghamella elegansPalmer-Brown, W., Dunne, B., Ortin, Y., Fox, M. A., Sandford, G., & Murphy, C. D. (2016). Biotransformation of fluorophenyl pyridine carboxylic acids by the model fungus Cunninghamella elegans. Xenobiotica, 47(9), 763-770. https://doi.org/10.1080/00498254.2016.1227109
- Sulfonyl-Substituted Heteroleptic Cyclometalated Iridium(III) Complexes as Blue Emitters for Solution-Processable Phosphorescent Organic Light-Emitting DiodesBenjamin, H., Zheng, Y., Batsanov, A., Fox, M., Al-Attar, H., Monkman, A., & Bryce, M. (2016). Sulfonyl-Substituted Heteroleptic Cyclometalated Iridium(III) Complexes as Blue Emitters for Solution-Processable Phosphorescent Organic Light-Emitting Diodes. Inorganic Chemistry, 55(17), 8612-8627. https://doi.org/10.1021/acs.inorgchem.6b01179
- Bis-Tridentate Iridium(III) Phosphors Bearing Functional 2-Phenyl-6-(imidazol-2-ylidene)pyridine and 2-(Pyrazol-3-yl)-6-phenylpyridine Chelates for Efficient OLEDsLin, J., Chau, N., Liao, J., Wong, W., Lu, C., Sie, Z., Chang, C., Fox, M., Low, P., Lee, G., & Chi, Y. (2016). Bis-Tridentate Iridium(III) Phosphors Bearing Functional 2-Phenyl-6-(imidazol-2-ylidene)pyridine and 2-(Pyrazol-3-yl)-6-phenylpyridine Chelates for Efficient OLEDs. Organometallics, 35(11), 1813-1824. https://doi.org/10.1021/acs.organomet.6b00205
- Induced europium CPL for the selective signalling of phosphorylated amino-acids and O-phosphorylated hexapeptidesNeil, E., Pal, R., Fox, M., & Parker, D. (2016). Induced europium CPL for the selective signalling of phosphorylated amino-acids and O-phosphorylated hexapeptides. Dalton Transactions, 45(20), 8355-8366. https://doi.org/10.1039/c6dt01212d
- Tethered N-heterocyclic carbene–carboranes: unique ligands that exhibit unprecedented and versatile coordination modes at rhodiumHolmes, J., Pask, C., Fox, M., & Willans, C. (2016). Tethered N-heterocyclic carbene–carboranes: unique ligands that exhibit unprecedented and versatile coordination modes at rhodium. Chemical Communications, 52(38), 6443-6446. https://doi.org/10.1039/c6cc01650b
- Low-melting molecular complexes: Part VII. 2,3-, 2,5- and 3,4-hexanediones and their molecular complexes with chloroformYufit, D., & Fox, M. (2016). Low-melting molecular complexes: Part VII. 2,3-, 2,5- and 3,4-hexanediones and their molecular complexes with chloroform. Structural Chemistry, 27(1), 9-15. https://doi.org/10.1007/s11224-015-0620-x
- Substituent Effects on the Fluorescence Properties of ortho-Carboranes: Unusual Emission Behaviour in C-(2′-Pyridyl)-ortho-carboranesBöhling, L., Brockhinke, A., Kahlert, J., Weber, L., Harder, R., Yufit, D., Howard, J., MacBride, J., & Fox, M. (2016). Substituent Effects on the Fluorescence Properties of ortho-Carboranes: Unusual Emission Behaviour in C-(2′-Pyridyl)-ortho-carboranes. European Journal of Inorganic Chemistry, 2016(3), 403-412. https://doi.org/10.1002/ejic.201501284
- Ir(III)-Based Phosphors with Bipyrazolate Ancillaries; Rational Design, Photophysics, and Applications in Organic Light-Emitting DiodesLiao, J., Chi, Y., Sie, Z., Ku, C., Chang, C., Fox, M., Low, P., Tseng, M., & Lee, G. (2015). Ir(III)-Based Phosphors with Bipyrazolate Ancillaries; Rational Design, Photophysics, and Applications in Organic Light-Emitting Diodes. Inorganic Chemistry, 54(22), 10811-10821. https://doi.org/10.1021/acs.inorgchem.5b01835
- An experimental and computational approach to understanding the reactions of acyl nitroso compounds in [4+2]-cycloadditionsChaiyaveij, D., Batsanov, A., Fox, M., Marder, T., & Whiting, A. (2015). An experimental and computational approach to understanding the reactions of acyl nitroso compounds in [4+2]-cycloadditions. Journal of Organic Chemistry, 80(19), 9518-9534. https://doi.org/10.1021/acs.joc.5b01470
- Chiral probe development for circularly polarised luminescence: comparative study of structural factors determining the degree of induced CPL with four heptacoordinate europium(III) complexesNeil, E., Fox, M., Pal, R., Pålsson, L., O’Sullivan, B., & Parker, D. (2015). Chiral probe development for circularly polarised luminescence: comparative study of structural factors determining the degree of induced CPL with four heptacoordinate europium(III) complexes. Dalton Transactions, 44(33), 14937-14951. https://doi.org/10.1039/c5dt02358k
- Challenging lanthanide relaxation theory: erbium and thulium complexes that show NMR relaxation rates faster than dysprosium and terbium analoguesFunk, A., Harvey, P., Finney, K., Fox, M., Kenwright, A., Rogers, N., Senanayake, P., & Parker, D. (2015). Challenging lanthanide relaxation theory: erbium and thulium complexes that show NMR relaxation rates faster than dysprosium and terbium analogues. Physical Chemistry Chemical Physics, 17(25), 16507-16511. https://doi.org/10.1039/c5cp02210j
- Syntheses and Structures of Buta-1,3-Diynyl Complexes from ‘On Complex’ Cross-Coupling ReactionsOerthel, M., Yufit, D., Fox, M., Bryce, M., & Low, P. (2015). Syntheses and Structures of Buta-1,3-Diynyl Complexes from ‘On Complex’ Cross-Coupling Reactions. Organometallics, 34(11), 2395-2405. https://doi.org/10.1021/om501186c
- Syntheses and Reductions of C-Dimesitylboryl-1,2-dicarba-closo-dodecaboranesKahlert, J., Böhling, L., Brockhinke, A., Stammler, H., Newmann, B., Rendina, L., Low, P., Weber, L., & Fox, M. (2015). Syntheses and Reductions of C-Dimesitylboryl-1,2-dicarba-closo-dodecaboranes. Dalton Transactions, 44(21), 9766-9781. https://doi.org/10.1039/c5dt00758e
- New donor–acceptor conjugates based on a trifluorenylporphyrin linked to a redox–switchable ruthenium unitMerhi, A., Zhang, X., Yao, D., Drouet, S., Mongin, O., Paul, F., Williams, J., Fox, M., & Paul-Roth, C. (2015). New donor–acceptor conjugates based on a trifluorenylporphyrin linked to a redox–switchable ruthenium unit. Dalton Transactions, 44(20), 9470-9485. https://doi.org/10.1039/c5dt00348b
- Near infrared-emitting tris-bidentate Os(II) phosphors: control of excited state characteristics and fabrication of OLEDsLiao, J., Chi, Y., Yeh, C., Kao, H., Chang, C., Fox, M., Low, P., & Lee, G. (2015). Near infrared-emitting tris-bidentate Os(II) phosphors: control of excited state characteristics and fabrication of OLEDs. Journal of Materials Chemistry C Materials for Optical and Electronic Devices, 3(19), 4910-4920. https://doi.org/10.1039/c5tc00204d
- Why are the {Cu4N4} rings in copper(I) phosphinimide clusters [Cu{μ-N=PR3}]4 (R = NMe3 or Ph) planar?Robinson, T., Price, R., Davidson, M., Fox, M., & Johnson, A. (2015). Why are the {Cu4N4} rings in copper(I) phosphinimide clusters [Cu{μ-N=PR3}]4 (R = NMe3 or Ph) planar?. Dalton Transactions, 44(12), 5611-5619. https://doi.org/10.1039/c5dt00255a
- Oligo(p-phenyleneethynylene) (OPE) Molecular Wires: Synthesis and Length Dependence of Photoinduced Charge Transfer in OPEs with Triarylamine and Diaryloxadiazole End GroupsLinton, K., Fox, M., Pålsson, L., & Bryce, M. (2015). Oligo(p-phenyleneethynylene) (OPE) Molecular Wires: Synthesis and Length Dependence of Photoinduced Charge Transfer in OPEs with Triarylamine and Diaryloxadiazole End Groups. Chemistry - A European Journal, 21(10), 3997-4007. https://doi.org/10.1002/chem.201406080
- Electronic Structure and Charge Transport Properties of a Series of 3,6-(Diphenyl)-s-tetrazine Derivatives: Are They Suitable Candidates for Molecular Electronics?Moral, M., García, G., Garzón, A., Granadino-Roldán, J., Fox, M., Yufit, D., Peñas, A., Melguizo, M., & Fernández-Gómez, M. (2014). Electronic Structure and Charge Transport Properties of a Series of 3,6-(Diphenyl)-s-tetrazine Derivatives: Are They Suitable Candidates for Molecular Electronics?. Journal of Physical Chemistry C, 118(46), 26427-26439. https://doi.org/10.1021/jp5049698
- Bimetallic Cyclometalated Iridium(III) Diastereomers with Non-Innocent Bridging Ligands for High-Efficiency Phosphorescent OLEDsZheng, Y., Batsanov, A., Fox, M., Al-Attar, H., Abdullah, K., Jankus, V., Bryce, M., & Monkman, A. (2014). Bimetallic Cyclometalated Iridium(III) Diastereomers with Non-Innocent Bridging Ligands for High-Efficiency Phosphorescent OLEDs. Angewandte Chemie International Edition, 53(43), 11616-11619. https://doi.org/10.1002/anie.201407475
- Studies on bis(1’-ortho-carboranyl)benzenes and bis(1’-ortho-carboranyl)biphenylsHarder, R., MacBride, J., Rivers, G., Yufit, D., Goeta, A., Howard, J., Wade, K., & Fox, M. (2014). Studies on bis(1’-ortho-carboranyl)benzenes and bis(1’-ortho-carboranyl)biphenyls. Tetrahedron, 70(34), 5182-5189. https://doi.org/10.1016/j.tet.2014.05.102
- Ultrafast Dynamics and Computational Studies on Diaminodicyanoquinodimethanes (DADQs)Szablewski, M., Fox, M., Dias, F., Namih, H., Snedden, E., King, S., Dai, D., & Pålsson, L. (2014). Ultrafast Dynamics and Computational Studies on Diaminodicyanoquinodimethanes (DADQs). Journal of Physical Chemistry B (Soft Condensed Matter and Biophysical Chemistry), 118(24), 6815-6828. https://doi.org/10.1021/jp411358d
- Mixed-Valence Ruthenium Complexes Rotating through a Conformational Robin–Day ContinuumParthey, M., Gluyas, J., Fox, M., Low , P., & Kaupp, M. (2014). Mixed-Valence Ruthenium Complexes Rotating through a Conformational Robin–Day Continuum. Chemistry - A European Journal, 20(23), 6895-6908. https://doi.org/10.1002/chem.201304947
- Crystal structures of the carborane dianions, [1,4-(PhCB10H10C)2C6H4]2- and [1,4-(PhCB10H10C)2C6F4]2-, and the stabilizing role of the para-phenylene unit on unusual 2n+3 SE clustersKahlert, J., Stammler, H., Neumann, B., Harder, R., Weber, L., & Fox, M. (2014). Crystal structures of the carborane dianions, [1,4-(PhCB10H10C)2C6H4]2- and [1,4-(PhCB10H10C)2C6F4]2-, and the stabilizing role of the para-phenylene unit on unusual 2n+3 SE clusters. Angewandte Chemie International Edition, 53(14), 3702-3705. https://doi.org/10.1002/anie.201310718
- On the ambiguity of 1,3,2-benzodiazaboroles as donor/acceptor functionalities in luminescent moleculesWeber, L., Halama, J., Hanke, K., Böhling, L., Brockhinke, A., Stammler, H., Neumann, B., & Fox, M. (2014). On the ambiguity of 1,3,2-benzodiazaboroles as donor/acceptor functionalities in luminescent molecules. Dalton Transactions, 43(8), 3347-3363. https://doi.org/10.1039/c3dt52836g
- 19F and 13C GIAO-NMR chemical shifts for the identification of perfluoro-quinoline and -isoquinoline derivativesFox, M., Pattison, G., Sandford, G., & Batsanov, A. (2013). 19F and 13C GIAO-NMR chemical shifts for the identification of perfluoro-quinoline and -isoquinoline derivatives. Journal of Fluorine Chemistry, 155, 62-71. https://doi.org/10.1016/j.jfluchem.2013.05.005
- C,C′-Bis(benzodiazaborolyl)dicarba-closo-dodecaboranes: Synthesis, structures, photophysics and electrochemistryWeber, L., Kahlert, J., Brockhinke, R., Böhling, L., Halama, J., Brockhinke, A., Stammler, H., Neumann, B., Nervi, C., Harder, R., & Fox, M. (2013). C,C′-Bis(benzodiazaborolyl)dicarba-closo-dodecaboranes: Synthesis, structures, photophysics and electrochemistry. Dalton Transactions, 42(30), 10982-10996. https://doi.org/10.1039/c3dt51125a
- A novel, efficient synthesis of N-aryl pyrroles via reaction of 1-boronodienes with arylnitroso compoundsTripoteau, F., Eberlin, L., Fox, M., Carboni, B., & Whiting, A. (2013). A novel, efficient synthesis of N-aryl pyrroles via reaction of 1-boronodienes with arylnitroso compounds. Chemical Communications, 49(47), 5414-5416. https://doi.org/10.1039/c3cc42227e
- Remarkable cage deboronation and rearrangement of a closo-1,12-dicarbadodecaborane to form a neutral nido-7,9-dicarbaundecaboraneIoppolo, J., Bhadbhade, M., Fox, M., & Rendina, L. (2013). Remarkable cage deboronation and rearrangement of a closo-1,12-dicarbadodecaborane to form a neutral nido-7,9-dicarbaundecaborane. Chemical Communications, 49(32), 3312-3314. https://doi.org/10.1039/c3cc41173g
- Electrochemical and spectroelectrochemical studies of C-benzodiazaborolyl-ortho-carboranesWeber, L., Kahlert, J., Boehling, L., Brockhinke, A., Stammler, H., Neumann, B., Harder, R., Low, P., & Fox, M. (2013). Electrochemical and spectroelectrochemical studies of C-benzodiazaborolyl-ortho-carboranes. Dalton Transactions, 42(6), 2266-2281. https://doi.org/10.1039/c2dt32378h
- Some Ruthenium Derivatives of Penta-1,4-diyn-3-oneBruce, M. I., Burgun, A., Fox, M. A., Jevric, M., Low, P. J., Nicholson, B. K., Parker Christian, R., Skelton, B. W., White, A. H., & Zaitseva, N. N. (2013). Some Ruthenium Derivatives of Penta-1,4-diyn-3-one. Organometallics, 32(11), 3286-3299. https://doi.org/10.1021/om400208q
- Syntheses, structures and redox properties of tris(pyrazolyl)borate-capped ruthenium vinyl complexesFarmer, J. D., Man, W. Y., Fox, M. A., Yufit, D. S., Howard, J. A., Hill, A. F., & Low, P. J. (2012). Syntheses, structures and redox properties of tris(pyrazolyl)borate-capped ruthenium vinyl complexes. Journal of Organometallic Chemistry, 721-722, 173-185. https://doi.org/10.1016/j.jorganchem.2012.09.001
- Carboranes as model hypercarbon systems; structural and bonding patterns in selected isoelectronic closo-borane and carborane systems; [BnHn](2-), [1-CBn-1Hn](-) and 1,n-C2Bn-2Hn (n=5, 6, 7, 10 or 12)Armstrong, D., Fox, M., & Wade, K. (2012). Carboranes as model hypercarbon systems; structural and bonding patterns in selected isoelectronic closo-borane and carborane systems; [BnHn](2-), [1-CBn-1Hn](-) and 1,n-C2Bn-2Hn (n=5, 6, 7, 10 or 12). Journal of Organometallic Chemistry, 721, 130-136. https://doi.org/10.1016/j.jorganchem.2012.07.047
- Reactions of 4-substituted tetrafluoropyridine derivatives with sulfur nucleophiles: SNAr and annelation processesFox, M. A., Sandford, G., Slater, R., Yufit, D. S., Howard, J. A., & Vong, A. (2012). Reactions of 4-substituted tetrafluoropyridine derivatives with sulfur nucleophiles: SNAr and annelation processes. Journal of Fluorine Chemistry, 143(SI), 148-154. https://doi.org/10.1016/j.jfluchem.2012.05.011
- Phosphine-alkene ligand-mediated alkyl-alkyl and alkyl-halide elimination processes from palladium(II)Tuxworth, L., Baiget, L., Phanopoulos, A., Metters, O. J., Batsanov, A. S., Fox, M. A., Howard, J. A., & Dyer, P. W. (2012). Phosphine-alkene ligand-mediated alkyl-alkyl and alkyl-halide elimination processes from palladium(II). Chemical Communications, 48(84), 10413-10415. https://doi.org/10.1039/c2cc35623f
- Diazaborolyl-boryl push–pull systems with ethynylene–arylene bridges as ‘turn-on’ fluoride sensorsWeber, L., Eickhoff, D., Kahlert, J., Boehling, L., Brockhinke, A., Stammler, H., Neumann, B., & Fox, M. A. (2012). Diazaborolyl-boryl push–pull systems with ethynylene–arylene bridges as ‘turn-on’ fluoride sensors. Dalton Transactions, 41(34), 10328-10346. https://doi.org/10.1039/c2dt30438d
- Luminescence Properties of C-Diazaborolyl-ortho-Carboranes as Donor-Acceptor SystemsWeber, L., Kahlert, J., Brockhinke, R., Boehling, L., Brockhinke, A., Stammler, H., Neumann, B., Harder, R., & Fox, M. (2012). Luminescence Properties of C-Diazaborolyl-ortho-Carboranes as Donor-Acceptor Systems. Chemistry - A European Journal, 18(27), 8347-8357. https://doi.org/10.1002/chem.201200390
- Experimental and Theoretical Studies on Organic D-π-A Systems Containing Three-Coordinate Boron Moieties as both π-Donor and π-AcceptorWeber, L., Eickhoff, D., Marder, T. B., Fox, M. A., Low, P. J., Dwyer, A. D., Tozer, D. J., Schwedler, S., Brockhinke, A., Stammler, H., & Neumann, B. (2012). Experimental and Theoretical Studies on Organic D-π-A Systems Containing Three-Coordinate Boron Moieties as both π-Donor and π-Acceptor. Chemistry - A European Journal, 18(5), 1369-1382. https://doi.org/10.1002/chem.201102059
- peri-Dimethylamino substituent effects on proton transfer at carbon in α-naphthylacetate esters: a model for mandelate racemaseDelley, R., Bandyopadhyay, S., Fox, M., Schliehe, C., Hodgson, D., Hollfelder, F., Kirby, A., & O’Donoghue, A. (2012). peri-Dimethylamino substituent effects on proton transfer at carbon in α-naphthylacetate esters: a model for mandelate racemase. Organic and Biomolecular Chemistry, 10(3), 590-596. https://doi.org/10.1039/c1ob06525d
- Colour tuning of blue electroluminescence using bipolar carbazole-oxadiazole molecules in single-active-layer organic light emitting devices (OLEDs)Linton, K., Fisher, A., Pearson, C., Fox, M., Pålsson, L., Bryce, M., & Petty, M. (2012). Colour tuning of blue electroluminescence using bipolar carbazole-oxadiazole molecules in single-active-layer organic light emitting devices (OLEDs). Journal of Materials Chemistry, 22(23), 11816-11825. https://doi.org/10.1039/c2jm31825c
- Platinum(II) Complexes of Cyclic Triphosphenium Ions: a P-31 NMR Spectroscopic and Computational StudyCoffer (nee Monks), P. K., Deng, R. M., Dillon, K. B., Fox, M. A., & Olivey, R. J. (2012). Platinum(II) Complexes of Cyclic Triphosphenium Ions: a P-31 NMR Spectroscopic and Computational Study. Inorganic Chemistry, 51(18), 9799-9808. https://doi.org/10.1021/ic3011663
- Dinuclear iridium(III) complexes of cyclometalated fluorenylpyridine ligands as phosphorescent dopants for efficient solution-processed OLEDsM’hamedi, A., Batsanov, A. S., Fox, M. A., Bryce, M. R., Abdullah, K., Al-Attar, H. A., & Monkman, A. P. (2012). Dinuclear iridium(III) complexes of cyclometalated fluorenylpyridine ligands as phosphorescent dopants for efficient solution-processed OLEDs. Journal of Materials Chemistry, 22(27), 13529-13540. https://doi.org/10.1039/c2jm31143g
- Colour tuning from green to red by substituent effects in phosphorescent tris-cyclometalated iridium(III) complexes of carbazole-based ligands: synthetic, photophysical, computational and high efficiency OLED studiesTavasli, M., Moore, T. N., Zheng, Y., Bryce, M. R., Fox, M. A., Griffiths, G. C., Jankus, V., Al-Attar, H. A., & Monkman, A. P. (2012). Colour tuning from green to red by substituent effects in phosphorescent tris-cyclometalated iridium(III) complexes of carbazole-based ligands: synthetic, photophysical, computational and high efficiency OLED studies. Journal of Materials Chemistry, 22(13), 6419-6428. https://doi.org/10.1039/c2jm15049b
- The synthesis, molecular and electronic structure of cyanovinylidene complexesLong, E. M., Brown, N. J., Man, W. Y., Fox, M. A., Yufit, D. S., Howard, J. A., & Low, P. J. (2012). The synthesis, molecular and electronic structure of cyanovinylidene complexes. Inorganica Chimica Acta, 380, 358-371. https://doi.org/10.1016/j.ica.2011.10.067
- Substitution of Tetracyanoethene by Ethynyl-Metal Complexes Gives Tricyanovinylethynyl (Tricyanobutenynyl) Derivatives: Syntheses, Protonation, and Addition of Metal-Ligand FragmentsBruce, M. I., Fox, M. A., Low, P. J., Nicholson, B. K., Parker, C. R., Patalinghug, W. C., Skelton, B. W., & White, A. H. (2012). Substitution of Tetracyanoethene by Ethynyl-Metal Complexes Gives Tricyanovinylethynyl (Tricyanobutenynyl) Derivatives: Syntheses, Protonation, and Addition of Metal-Ligand Fragments. Organometallics, 31(7), 2639-2657. https://doi.org/10.1021/om2007503
- Synthesis and Characterization of Dithia[3.3]paracyclophane-Bridged Binuclear Ruthenium Vinyl and Alkynyl ComplexesXia, J., Man, W. Y., Zhu, X., Zhang, C., Jin, G., Schauer, P. A., Fox, M. A., Yin, J., Yu, G., Low, P. J., & Liu, S. H. (2012). Synthesis and Characterization of Dithia[3.3]paracyclophane-Bridged Binuclear Ruthenium Vinyl and Alkynyl Complexes. Organometallics, 31(15), 5321-5333. https://doi.org/10.1021/om300338j
- 1,2-Carbagerma-closo-dodecaborate as a Germanium Ligand in Coordination Chemistry - Synthesis, Structure and ReactivityWagenpfeil, A., Nickl, C., Schubert, H., Eichele, K., Fox, M. A., & Wesemann, L. (2011). 1,2-Carbagerma-closo-dodecaborate as a Germanium Ligand in Coordination Chemistry - Synthesis, Structure and Reactivity. European Journal of Inorganic Chemistry, 2011(22), 3349-3356. https://doi.org/10.1002/ejic.201100310
- Ligand redox non-innocent behaviour in ruthenium complexes of ethynyl tolansKhairul, W., Fox, M., Schauer, P., Albesa-Jove, D., Yufit, D., Howard, J., & Low, P. (2011). Ligand redox non-innocent behaviour in ruthenium complexes of ethynyl tolans. Inorganica Chimica Acta, 374(1), 461-471. https://doi.org/10.1016/j.ica.2011.02.043
- Highly Efficient, Solution-Processed, Single-Layer, Electrophosphorescent Diodes and the Effect of Molecular Dipole MomentAl-Attar, H., Griffiths, G., Moore, T., Tavasli, M., Fox, M., Bryce, M., & Monkman, A. (2011). Highly Efficient, Solution-Processed, Single-Layer, Electrophosphorescent Diodes and the Effect of Molecular Dipole Moment. Advanced Functional Materials, 21(12), 2376-2382. https://doi.org/10.1002/adfm.201100324
- Molybdenum Complexes of C,C-Bis(ethynyl)carboranes: Design, Synthesis, and Study of a Weakly Coupled Mixed-Valence CompoundBrown, N. J., Lancashire, H. N., Fox, M. A., Collison, D., Edge, R., Yufit, D. S., Howard, J. A., Whiteley, M. W., & Low, P. J. (2011). Molybdenum Complexes of C,C-Bis(ethynyl)carboranes: Design, Synthesis, and Study of a Weakly Coupled Mixed-Valence Compound. Organometallics, 30(4), 884-894. https://doi.org/10.1021/om1010353
- Simultaneous Bridge-Localized and Mixed-Valence Character in Diruthenium Radical Cations Featuring Diethynylaromatic Bridging LigandsFox, M., Guennic, B., Roberts, R., Brue, D., Yufit, D., Howard, J., Manca, G., Halet, J., Hartl, F., & Low, P. (2011). Simultaneous Bridge-Localized and Mixed-Valence Character in Diruthenium Radical Cations Featuring Diethynylaromatic Bridging Ligands. Journal of the American Chemical Society, 133(45), 18433-18446. https://doi.org/10.1021/ja207827m
- The electronic structures of diruthenium complexes containing an oligo(phenylene ethynylene) bridging ligand, and some related molecular structuresKhairul, W. M., Fox, M. A., Schauer, P. A., Yufit, D. S., Albesa-Jové, D., Howard, J. A., & Low, P. J. (2010). The electronic structures of diruthenium complexes containing an oligo(phenylene ethynylene) bridging ligand, and some related molecular structures. Dalton Transactions, 39(48), 11605-11615. https://doi.org/10.1039/c0dt00809e
- Deboronation and Deprotonation of ortho-Carborane with N-Heterocyclic CarbenesWillans, C., Kilner, C., & Fox, M. (2010). Deboronation and Deprotonation of ortho-Carborane with N-Heterocyclic Carbenes. Chemistry - A European Journal, 16(35), 10644-10648. https://doi.org/10.1002/chem.201001730
- Luminescent Platinum(II) Complexes Containing Cyclometallated Diaryl Ketimine Ligands: Synthesis, Photophysical and Computational PropertiesPandya, S., Moss, K., Bryce, M., Batsanov, A., Fox, M., Jankus, V., Al Attar, H., & Monkman, A. (2010). Luminescent Platinum(II) Complexes Containing Cyclometallated Diaryl Ketimine Ligands: Synthesis, Photophysical and Computational Properties. European Journal of Inorganic Chemistry, 2010(13), 1963-1972. https://doi.org/10.1002/ejic.200901159
- Some reactions of an eta(3)-tetracyanobutadienyl-ruthenium complexBruce, M. I., Fox, M. A., Low, P. J., Skelton, B. W., & Zaitseva, N. N. (2010). Some reactions of an eta(3)-tetracyanobutadienyl-ruthenium complex. Dalton Transactions, 39(15), 3759-3770. https://doi.org/10.1039/b921324d
- Syntheses and molecular structures of some tricobalt carbonyl clusters containing 2,4,6-trimethyl-1,3,5-trithianeBruce, M., Zaitseva, N., Skelton, B., White, A., Fox, M., & Low, P. (2010). Syntheses and molecular structures of some tricobalt carbonyl clusters containing 2,4,6-trimethyl-1,3,5-trithiane. Dalton Transactions, 39(5), 1222-1234. https://doi.org/10.1039/b909708b
- Tuning the Intramolecular Charge Transfer Emission from Deep Blue to Green in Ambipolar Systems Based On Dibenzothiophene S S-Dioxide by Manipulation of Conjugation and Strength of the Electron Donor UnitsMoss, K., Bourdakos, K., Bhalla, V., Kamtekar, K., Bryce, M., Fox, M., Vaughan, H., Dias, F., & Monkman, A. (2010). Tuning the Intramolecular Charge Transfer Emission from Deep Blue to Green in Ambipolar Systems Based On Dibenzothiophene S S-Dioxide by Manipulation of Conjugation and Strength of the Electron Donor Units. Journal of Organic Chemistry, 75(20), 6771-6781. https://doi.org/10.1021/jo100898a
- The syntheses and structures of mono- and di-bromovinylidenes.Brown, N. J., Fox, M. A., Smith, M. E., Yufit, D. S., Howard, J. A., & Low, P. J. (2009). The syntheses and structures of mono- and di-bromovinylidenes. Journal of Organometallic Chemistry, 694(25), 4042-4048. https://doi.org/10.1016/j.jorganchem.2009.08.027
- From Cyclic Iminophosphoranes to -Conjugated MaterialsSmith, D., Batsanov, A., Fox, M., Beeby, A., Apperley, D., Howard, J., & Dyer, P. (2009). From Cyclic Iminophosphoranes to -Conjugated Materials. Angewandte Chemie International Edition, 48(48), 9109-9113. https://doi.org/10.1002/anie.200904219
- Structural, spectroscopic, electrochemical and computational studies of C,C '-diaryl-ortho-carboranes, 1-(4-XC6H4)-2-Ph-1,2-C2B10H10 (X = H, F, OMe, NMe2, NH2, OH and O-)Fox, M., Nervi, C., Crivello, A., Batsanov, A., Howard, J., Wade, K., & Low, P. (2009). Structural, spectroscopic, electrochemical and computational studies of C,C ’-diaryl-ortho-carboranes, 1-(4-XC6H4)-2-Ph-1,2-C2B10H10 (X = H, F, OMe, NMe2, NH2, OH and O-). Journal of Solid State Electrochemistry, 13(10), 1483-1495. https://doi.org/10.1007/s10008-008-0686-0
- Noninnocent Ligand Behavior in Diruthenium Complexes Containing a 1,3-Diethynylbenzene BridgeFox, M., Farmer, J., Roberts, R., Humphrey, M., & Low, P. (2009). Noninnocent Ligand Behavior in Diruthenium Complexes Containing a 1,3-Diethynylbenzene Bridge. Organometallics, 28(17), 5266-5269. https://doi.org/10.1021/om900200n
- Trends in ortho-carboranes 1-X-2-R-1,2-C2B10H10 (R = Ph, Me) bearing an exo-CN-bonded substituent group (X = NO, N=NR ' or NHR '')Fox, M., Peace, R., Clegg, W., Elsegood, M., & Wade, K. (2009). Trends in ortho-carboranes 1-X-2-R-1,2-C2B10H10 (R = Ph, Me) bearing an exo-CN-bonded substituent group (X = NO, N=NR ’ or NHR ’’). Polyhedron, 28(12), 2359-2370. https://doi.org/10.1016/j.poly.2009.04.041
- A simple synthesis of trans-RuCl(C CR)(dppe)(2) complexes and representative molecular structuresFox, M. A., Harris, J. E., Heider, S., Pérez-Gregorio, V., Zakrzewska, M. E., Farmer, J. D., Yufit, D. S., Howard, J. A., & Low, P. J. (2009). A simple synthesis of trans-RuCl(C CR)(dppe)(2) complexes and representative molecular structures. Journal of Organometallic Chemistry, 694(15), 2350-2358. https://doi.org/10.1016/j.jorganchem.2009.03.033
- Synthetic, structural, photophysical and computational studies on 2-arylethynyl-1,3,2-diazaborolesWeber, L., Werner, V., Fox, M. A., Marder, T. B., Schwedler, S., Brockhinke, A., Hans-Georg, S., & Neumann, B. (2009). Synthetic, structural, photophysical and computational studies on 2-arylethynyl-1,3,2-diazaboroles. Dalton Transactions, 2009(15), 2823-2831. https://doi.org/10.1039/b821208b
- New synthetic and structural studies on nitroso-ortho-carboranes RCB10H10CNO and bis(ortho-carboranyl)amines (RCB10H10C)(2)NH (R = Ph or Me)Fox, M., MacBride, J., Peace, R., Clegg, W., Elsegood, M., & Wade, K. (2009). New synthetic and structural studies on nitroso-ortho-carboranes RCB10H10CNO and bis(ortho-carboranyl)amines (RCB10H10C)(2)NH (R = Ph or Me). Polyhedron, 28(4), 789-795. https://doi.org/10.1016/j.poly.2008.12.014
- Transition metal alkynyl complexes by transmetallation from Au(C[triple bond, length as m-dash]CAr)(PPh3) (Ar = C6H5 or C6H4Me-4)Khairul, W. M., Fox, M. A., Zaitseva, N. N., Gaudio, M., Yufit, D. S., Skelton, B. W., White, A. H., Howard, J. A., Bruce, M. I., & Low, P. J. (2009). Transition metal alkynyl complexes by transmetallation from Au(C[triple bond, length as m-dash]CAr)(PPh3) (Ar = C6H5 or C6H4Me-4). Dalton Transactions, 2009(4), 610-620. https://doi.org/10.1039/b809960j
- DFT studies of the σ-donor/π-acceptor properties of [SnCB10H11]– and its relationship to [SnCl3]–, CO, PF3, [SnB11H11]2–, SnC2B9H11, and related SnC2BnHn+2 compoundsFox, M. A., Marder, T. B., & Wesemann, L. (2009). DFT studies of the σ-donor/π-acceptor properties of [SnCB10H11]– and its relationship to [SnCl3]–, CO, PF3, [SnB11H11]2–, SnC2B9H11, and related SnC2BnHn+2 compounds. Canadian Journal of Chemistry, 87(1), 63-71. https://doi.org/10.1139/v08-081
- EXPERIMENTAL AND COMPUTED DIPOLE MOMENTS IN DONOR-BRIDGE-ACCEPTOR SYSTEMS WITH p-PHENYLENE AND p-CARBORANEDIYL BRIDGESFox, M. (2009). EXPERIMENTAL AND COMPUTED DIPOLE MOMENTS IN DONOR-BRIDGE-ACCEPTOR SYSTEMS WITH p-PHENYLENE AND p-CARBORANEDIYL BRIDGES. Collection of Czechoslovak Chemical Communications., 74(1), 131-146. https://doi.org/10.1135/cccc2008154
- Some transition metal complexes derived from mono- and di-ethynyl perfluorobenzenes.Armitt, D. J., Bruce, M. I., Gaudio, M., Zaitseva, N. N., Skelton, B. W., White, A. H., Le Guennic, B., Halet, J., Fox, M. A., Roberts, R. L., Hartl, F., & Low, P. J. (2008). Some transition metal complexes derived from mono- and di-ethynyl perfluorobenzenes. Dalton Transactions, 2008(47), 6763-6775. https://doi.org/10.1039/b808798a
- Facile photoinduced charge separation through a cyanoacetylide bridge in a heterobimetallic Fe(ii)–Re(i) complexSmith, M. E., Flynn, E. L., Fox, M. A., Trottier, A., Wrede, E., Yufit, D. S., Howard, J. A., Ronayne, K. L., Towrie, M., Parker, A. W., Hartl, F., & Low, P. J. (2008). Facile photoinduced charge separation through a cyanoacetylide bridge in a heterobimetallic Fe(ii)–Re(i) complex. Chemical Communications, 2008(44), 5845-5847. https://doi.org/10.1039/b811357b
- Ruthenium complexes of C,C '-bis(ethynyl)carboranes: An investigation of electronic interactions mediated by spherical pseudo-aromatic spacersFox, M., Roberts, R., Baines, T., Le Guennic, B., Halet, J., Hartl, F., Yufit, D., Albesa-Jové, D., Howard, J., & Low, P. (2008). Ruthenium complexes of C,C ’-bis(ethynyl)carboranes: An investigation of electronic interactions mediated by spherical pseudo-aromatic spacers. Journal of the American Chemical Society, 130(11), 3566-3578. https://doi.org/10.1021/ja0779755
- N-Phosphino-amidines and -guanidines: synthesis, structure and P,N-chelate chemistry.Baiget, L., Batsanov, A. S., Dyer, P. W., Fox, M. A., Hanton, M. J., Howard, J. A., Lane, P. K., & Solomon, S. A. (2008). N-Phosphino-amidines and -guanidines: synthesis, structure and P,N-chelate chemistry. Dalton Transactions, 2008(8), 1043-1054. https://doi.org/10.1039/b715736c
- The preparation and characterisation of ruthenium cyanovinylidene complexes.Brown, N. J., Eckert, P. K., Fox, M. A., Yufit, D. S., Howard, J. A., & Low, P. J. (2008). The preparation and characterisation of ruthenium cyanovinylidene complexes. Dalton Transactions, 2008(4), 433-436. https://doi.org/10.1039/b714274a
- Quenched gas-phase reactions of tetraborane(10), B4H10, with substituted alkynes: new nido-dicarbapentaboranes and arachno-monocarbapentaboranesFox, M. A., Greatrex, R., & Nikrahi, A. (2008). Quenched gas-phase reactions of tetraborane(10), B4H10, with substituted alkynes: new nido-dicarbapentaboranes and arachno-monocarbapentaboranes. Dalton Transactions, 2008(5), 676-684. https://doi.org/10.1039/b715105e
- Spectroscopic properties and electronic structures of 17-electron half-sandwich ruthenium acetylide complexes, [Ru(CCAr)(L2)Cp′]+ (Ar=phenyl, p-tolyl, 1-naphthyl, 9-anthryl; L2=(PPh3)2, Cp′=Cp; L2=dppe; Cp′=Cp∗).Fox, M. A., Roberts, R. L., Khairul, W. M., Hartl, F., & Low, P. J. (2007). Spectroscopic properties and electronic structures of 17-electron half-sandwich ruthenium acetylide complexes, [Ru(CCAr)(L2)Cp′]+ (Ar=phenyl, p-tolyl, 1-naphthyl, 9-anthryl; L2=(PPh3)2, Cp′=Cp; L2=dppe; Cp′=Cp∗). Journal of Organometallic Chemistry, 692(15), 3277-3290. https://doi.org/10.1016/j.jorganchem.2007.03.042
- Carborane radical anions: spectroscopic and electronic properties of a carborane radical anion with a 2n + 3 skeletal electron countFox, M., Nervi, C., Crivello, A., & Low, P. (2007). Carborane radical anions: spectroscopic and electronic properties of a carborane radical anion with a 2n + 3 skeletal electron count. Chemical Communications, 23, 2372-2374. https://doi.org/10.1039/b700110j
- Elemental fluorine - Part 20. Direct fluorination of deactivated aromatic systems using microreactor techniquesFox, M. (2007). Elemental fluorine - Part 20. Direct fluorination of deactivated aromatic systems using microreactor techniques. Journal of Fluorine Chemistry, 128(1), 29-33. https://doi.org/10.1016/j.jfluchem.2006.09.010
- Improved syntheses of bis(ethynyl)-para-carboranes, 1,12-(RC C)(2)-1,12-C2B10H10 and 1,10-(RC equivalent to C)(2)-1,10-C2B8H8 (R = H or Me3Si).Fox, M., Baines, T., Albesa-Jove, D., Howard, J., & Low, P. (2006). Improved syntheses of bis(ethynyl)-para-carboranes, 1,12-(RC C)(2)-1,12-C2B10H10 and 1,10-(RC equivalent to C)(2)-1,10-C2B8H8 (R = H or Me3Si). Journal of Organometallic Chemistry, 691(18), 3889-3894. https://doi.org/10.1016/j.jorganchem.2006.05.044
- Synthetic and structural studies on C-ethynyl- and C-bromo-carboranes.Fox, M., Cameron, A., Low, P., Paterson, M., Batsanov, A., Goeta, A., Rankin, D., Robertson, H., & Schirlin, J. (2006). Synthetic and structural studies on C-ethynyl- and C-bromo-carboranes. Dalton Transactions, 29, 3544-3560. https://doi.org/10.1039/b517538k
- Reactions of Icosahedral Carboranes with Iminotris(dimethylamino)Phosphorane HNP(NMe2)3: a Deboronation Intermediate nido-C2B10H12·N(H)P(NMe2)3, Deboronation Reactions and Hydrogen-bonded Closo-carborane SystemsBatsanov, A. S., Copley, R. C., Davidson, M. G., Fox, M. A., Hibbert, T. G., Howard, J. A., & Wade, K. (2006). Reactions of Icosahedral Carboranes with Iminotris(dimethylamino)Phosphorane HNP(NMe2)3: a Deboronation Intermediate nido-C2B10H12·N(H)P(NMe2)3, Deboronation Reactions and Hydrogen-bonded Closo-carborane Systems. Journal of Cluster Science, 17(1), 119-137. https://doi.org/10.1007/s10876-005-0042-9
- Preparative and structural studies on sulfur-linked carborane icosahedra: 2-phenyl-ortho-carboranyl-sulfur systems (2-Ph-1,2-C2B10H10)(2)X (X = S, S-2 or SO), and ortho-carboran-di-yl systems (1,2-C2B10H10Y)(2) (Y = S or SO)Batsanov, A., Clegg, W., Copley, R., Fox, M., Gill, W., Grimditch, R., Hibbert, T., Howard, J., MacBride, J., & Wade, K. (2006). Preparative and structural studies on sulfur-linked carborane icosahedra: 2-phenyl-ortho-carboranyl-sulfur systems (2-Ph-1,2-C2B10H10)(2)X (X = S, S-2 or SO), and ortho-carboran-di-yl systems (1,2-C2B10H10Y)(2) (Y = S or SO). Polyhedron, 25(2), 300-306. https://doi.org/10.1016/j.poly.2005.06.046
- Electronic interactions in bridged bis(cluster) assemblies - a comparison of para-CB10H10C, para-C6H4 and C-4 bridgesLe Guennic, B., Costuas, K., Halet, J., Nervi, C., Paterson, M., Fox, M., Roberts, R., Albesa-Jove, D., Puschmann, H., Howard, J., & Low, P. (2005). Electronic interactions in bridged bis(cluster) assemblies - a comparison of para-CB10H10C, para-C6H4 and C-4 bridges. Comptes Rendus Chimie, 8(11-12), 1883-1896. https://doi.org/10.1016/j.crci.2005.03.016
- Elemental fluorine - Part 18. Selective direct fluorination of 1,3-ketoesters and 1,3-diketones using gas/liquid microreactor technologyChambers, R., Fox, M., & Sandford, G. (2005). Elemental fluorine - Part 18. Selective direct fluorination of 1,3-ketoesters and 1,3-diketones using gas/liquid microreactor technology. Lab on a Chip, 5(10), 1132-1139. https://doi.org/10.1039/b504675k
- Versatile gas/liquid microreactors for industryChambers, R., Fox, M., Holling, D., Nakano, T., Okazoe, T., & Sandford, G. (2005). Versatile gas/liquid microreactors for industry. Chemical Engineering and Technology, 28(3), 344-352. https://doi.org/10.1002/ceat.200407123
- Gas-phase electron diffraction studies of the icosahedral carbaboranes, ortho-, meta- and para-C2B10H12Turner, A. R., Robertson, H. E., Borisenko, K. B., Rankin, D. W. H., & Fox, M. A. (2005). Gas-phase electron diffraction studies of the icosahedral carbaboranes, ortho-, meta- and para-C2B10H12. Dalton Transactions, 2005(7), 1310-1318. https://doi.org/10.1039/B418276F
- Elemental fluorine - Part 16. Versatile thin-film gas-liquid multi-channel microreactors for effective scale-outChambers, R., Fox, M., Holling, D., Nakano, T., Okazoe, T., & Sandford, G. (2005). Elemental fluorine - Part 16. Versatile thin-film gas-liquid multi-channel microreactors for effective scale-out. Lab on a Chip, 5(2), 191-198. https://doi.org/10.1039/b416400h
- Sulfur, tin and gold derivatives of 1-(2′-pyridyl)-ortho-carborane, 1-R-2-X-1,2-C2B10H10 (R = 2′-pyridyl, X = SH, SnMe3 or AuPPh3)Batsanov, A., Fox, M., Hibbert, T., Howard, J., Kivekäs, R., Laromaine, A., Sillanpää, R., Viñas, C., & Wade, K. (2004). Sulfur, tin and gold derivatives of 1-(2′-pyridyl)-ortho-carborane, 1-R-2-X-1,2-C2B10H10 (R = 2′-pyridyl, X = SH, SnMe3 or AuPPh3). Dalton Transactions, 2004(22), 3822-3828. https://doi.org/10.1039/b411099d
- Exo-π-bonding to an ortho-carborane hypercarbon atom: systematic icosahedral cage distortions reflected in the structures of the fluoro-, hydroxy- and amino-carboranes, 1-X-2-Ph-1,2-C2B10H10 (X = F, OH or NH2) and related anionsBoyd, L., Clegg, W., Copley, R., Davidson, M., Fox, M., Hibbert, T., Howard, J., Mackinnon, A., Peace, R., & Wade, K. (2004). Exo-π-bonding to an ortho-carborane hypercarbon atom: systematic icosahedral cage distortions reflected in the structures of the fluoro-, hydroxy- and amino-carboranes, 1-X-2-Ph-1,2-C2B10H10 (X = F, OH or NH2) and related anions. Dalton Transactions, 2004(17), 2786-2799. https://doi.org/10.1039/b406422d
- Cage C---H...X interactions in solid-state structures of icosahedral carboranesFox, M., & Hughes, A. (2004). Cage C---H...X interactions in solid-state structures of icosahedral carboranes. Coordination Chemistry Reviews, 248(5-6), 457-476. https://doi.org/10.1016/j.ccr.2003.10.002
- Two contrasting ethynyl hydroboration pathways in the formation of a novel tris-hydroboration product from reaction of dimesitylborane with 2,5-diethynylpyridineEntwistle, C. D., Batsanov, A. S., Howard, J. A. K., Fox, M. A., & Marder, T. B. (2004). Two contrasting ethynyl hydroboration pathways in the formation of a novel tris-hydroboration product from reaction of dimesitylborane with 2,5-diethynylpyridine. Chemical Communications, 2004(6), 702-703. https://doi.org/10.1039/B316250H
- The synthesis and molecular and crystal structures of 1-methyl-2-carboxy-1,2-dicarba-closo-dodecaborane(12), 1-phenyl-2-carboxy-1,2-dicarba-closo-dodecaborane(12) and 1-phenyl-2-benzoyl-1,2-dicarba-closo-dodecaborane(12)Venkatasubramanian, U., Donohoe, D., Ellis, D., Giles, B., Macgregor, S., Robertson, S., Rosair, G., Welch, A., Batsanov, A., Boyd, L., Copley, R., Fox, M., Howard, J., & Wade, K. (2004). The synthesis and molecular and crystal structures of 1-methyl-2-carboxy-1,2-dicarba-closo-dodecaborane(12), 1-phenyl-2-carboxy-1,2-dicarba-closo-dodecaborane(12) and 1-phenyl-2-benzoyl-1,2-dicarba-closo-dodecaborane(12). Polyhedron, 23(4), 629-636.
- Gas-phase electron diffraction studies on two 11-vertex dicarbaboranes, closo-2,3-C2B9H11 and nido-2,9-C2B9H13Fox, M. (2004). Gas-phase electron diffraction studies on two 11-vertex dicarbaboranes, closo-2,3-C2B9H11 and nido-2,9-C2B9H13. Inorganic Chemistry, 43(17), 5387-5392.
- Evolving patterns in boron cluster chemistryFox, M., & Wade, K. (2003). Evolving patterns in boron cluster chemistry. Pure and Applied Chemistry, 75(9), 1315-1323. https://doi.org/10.1351/pac200375091315
- Dimesitylborane monomer-dimer equilibrium in solution, and the solid-state structure of the dimer by single crystal neutron and X-ray diffraction.Entwistle, C., Marder, T., Smith, P., Howard, J., Fox, M., & Mason, S. (2003). Dimesitylborane monomer-dimer equilibrium in solution, and the solid-state structure of the dimer by single crystal neutron and X-ray diffraction. Journal of Organometallic Chemistry, 680(1-2), 165-172. https://doi.org/10.1016/s0022-328x%2803%2900316-4
- Big macrocyclic assemblies of carboranes (big MACs): synthesis and crystal structure of a macrocyclic assembly of four carboranes containing alternate ortho- and meta-carborane icosahedra linked by para-phenylene units.Fox, M., Howard, J., MacBride, J., Mackinnon, A., & Wade, K. (2003). Big macrocyclic assemblies of carboranes (big MACs): synthesis and crystal structure of a macrocyclic assembly of four carboranes containing alternate ortho- and meta-carborane icosahedra linked by para-phenylene units. Journal of Organometallic Chemistry, 680(1-2), 155-164. https://doi.org/10.1016/s0022-328x%2803%2900313-9
- Synthesis and crystal structure of an assembly of three ortho-carborane cages linked via para-phenylene units: effect of aryl orientation on cage C-C bond lengths in C-aryl-ortho-carboranesAlekseyeva, E., Fox, M., Howard, J., MacBride, J., & Wade, K. (2003). Synthesis and crystal structure of an assembly of three ortho-carborane cages linked via para-phenylene units: effect of aryl orientation on cage C-C bond lengths in C-aryl-ortho-carboranes. Applied Organometallic Chemistry, 17(6-7), 499-508. https://doi.org/10.1002/aoc.467
- 9,12-Diiodo-1,2-dicarba-closo-dodecaborane(12)Batsanov, A., Fox, M., Howard, J., Hughes, A., Johnson, A., & Martindale, S. (2003). 9,12-Diiodo-1,2-dicarba-closo-dodecaborane(12). Acta Crystallographica Section C: Structural Chemistry, 59(2), O74-O76. https://doi.org/10.1107/s0108270102023582
- Alkynyl gold(I) rigid-rod molecules from 1,12-bis(ethynyl)-1,12-dicarba-closo-dodecaborane(12)Fox, M. (2003). Alkynyl gold(I) rigid-rod molecules from 1,12-bis(ethynyl)-1,12-dicarba-closo-dodecaborane(12). Organometallics, 22(23), 4792-4797.
- Synthesis and characterisation of some new boron compounds containing the 2,4,6-(CF₃)₃C₆H₂(fluoromes = Ar), 2,6-(CF₃)₂C₆H₃ (fluoroxyl = Ar '), or 2,4-(CF₃)₂C₆H₃ (Ar '') ligandsCornet, S., Dillon, K., Entwistle, C., Fox, M., Goeta, A., Goodwin, H., Marder, T., & Thompson, A. (2003). Synthesis and characterisation of some new boron compounds containing the 2,4,6-(CF₃)₃C₆H₂(fluoromes = Ar), 2,6-(CF₃)₂C₆H₃ (fluoroxyl = Ar ’), or 2,4-(CF₃)₂C₆H₃ (Ar ’’) ligands. Dalton Transactions, 2003(23), 4395-4405. https://doi.org/10.1039/b309820f
- Intra- and inter-molecular carboranyl C-H center dot center dot center dot N hydrogen bonds in pyridyl-containing ortho-carboranesAlekseyeva, E. S., Batsanov, A. S., Boyd, L. A., Fox, M. A., Hibbert, T. G., Howard, J. A. K., MacBride, J. A. H., Mackinnon, A., & Wade, K. (2003). Intra- and inter-molecular carboranyl C-H center dot center dot center dot N hydrogen bonds in pyridyl-containing ortho-carboranes. Dalton Transactions, 2003(3), 475-482. https://doi.org/10.1039/B209931D
- Unexpected formation of new fluoroboranes from the reaction of NMe4B3H8 with BF3 and MeC CH: exo-2-FB4H9 and trans-MeCH CHBF2Fox, M. A., Greatrex, R., & Ormsby, D. L. (2002). Unexpected formation of new fluoroboranes from the reaction of NMe4B3H8 with BF3 and MeC CH: exo-2-FB4H9 and trans-MeCH CHBF2. Chemical Communications, 2002(18), 2052-2053. https://doi.org/10.1039/B206102C
- A new nido-5-vertex cluster, phosphacarba-nido-pentaborane, 2-Bu-t-1,2-PCB3H5Condick, P. N., Fox, M. A., Greatrex, R., Jones, C., & Ormsby, D. L. (2002). A new nido-5-vertex cluster, phosphacarba-nido-pentaborane, 2-Bu-t-1,2-PCB3H5. Chemical Communications, 14, 1448-1449. https://doi.org/10.1039/B204409A
- Synthesis of a new boron carbonitride with a B4C-like structure from the thermolysis of N-alkylated borazinesBrydson, R., Daniels, H., Fox, M. A., Greatrex, R., & Workman, C. (2002). Synthesis of a new boron carbonitride with a B4C-like structure from the thermolysis of N-alkylated borazines. Chemical Communications, 2002(7), 718-719. https://doi.org/10.1039/B110856P
- Carbon-boron-nitrogen alloys from borazarene-derived mesophase pitches.Westwood, A., Brydson, R., Coult, R., Fox, M., Rand, B., & Wade, K. (2002). Carbon-boron-nitrogen alloys from borazarene-derived mesophase pitches. Carbon, 40(12), 2157-2167. https://doi.org/10.1016/s0008-6223%2802%2900064-7
- Crystal and molecular structures of the nido-carborane anions, 7,9- and 2,9 C₂B₉H₁₂Fox, M., Goeta, A., Hughes, A., & Johnson, A. (2002). Crystal and molecular structures of the nido-carborane anions, 7,9- and 2,9 C₂B₉H₁₂. Dalton Transactions, 10, 2132-2141. https://doi.org/10.1039/b108937d
- Do the discrete dianions C2B9H112- exist? Characterisation of alkali metal salts of the 11-vertex nido dicarboranes, C2B9H112-, in solutionFox, M., Hughes, A., Johnson, A., & Paterson, M. (2002). Do the discrete dianions C2B9H112- exist? Characterisation of alkali metal salts of the 11-vertex nido dicarboranes, C2B9H112-, in solution. Dalton Transactions., 2002(9), 2009-2019. https://doi.org/10.1039/b109804g
- Solution and solid-state structure of the anion [Ag-2closo-CB11H12(4)](2-)Fox, M. (2002). Solution and solid-state structure of the anion [Ag-2closo-CB11H12(4)](2-). Inorganic Chemistry, 41(17), 4567-4573.
- A convenient cyanide-free "one-pot" synthesis of nido-Me3N-7-CB10H12 and nido-7-CB10H13-Batsanov, A. S., Fox, M. A., Goeta, A. E., Howard, J. A. K., Hughes, A. K., & Malget, J. M. (2002). A convenient cyanide-free "one-pot" synthesis of nido-Me3N-7-CB10H12 and nido-7-CB10H13-. Dalton Transactions., 2002(13), 2624-2631. https://doi.org/10.1039/B200930G
- Cage-closing reactions of the nido-carborane anion 7,9-C2B9H12− and derivatives; formation of neutral 11-vertex carboranes by acidification.Fox, M., Hughes, A., & Malget, J. (2002). Cage-closing reactions of the nido-carborane anion 7,9-C2B9H12− and derivatives; formation of neutral 11-vertex carboranes by acidification. Dalton Transactions., 2002(18), 3505-3517. https://doi.org/10.1039/b203920f
- Model compounds and monomers for phenylene ether carboranylene ketone (PECK) polymer synthesis: preparation and characterization of boron-arylated ortho-carboranes bearing carboxyphenyl, phenoxyphenyl or benzoylphenyl substituentsFox, M., & Wade, K. (2002). Model compounds and monomers for phenylene ether carboranylene ketone (PECK) polymer synthesis: preparation and characterization of boron-arylated ortho-carboranes bearing carboxyphenyl, phenoxyphenyl or benzoylphenyl substituents. Journal of Materials Chemistry, 12(5), 1301-1306.
- Halogenation of tris(amido)tantalacarboranes with dihalomethanes CH2X2 (X = Cl, Br)Fox, M., Goeta, A., Hughes, A., Malget, J., & Wade, K. (2002). Halogenation of tris(amido)tantalacarboranes with dihalomethanes CH2X2 (X = Cl, Br). Collection of Czechoslovak Chemical Communications., 67(6), 791-807.
- Electrochemical evidence for electronic interactions through the para-carborane skeleton in the novel tricluster [{Co2C2(SiMe3)(CO)4(dppm)}2(μ-CB10H10C)]Fox, M., Paterson, M., Nervi, C., Galeotti, F., Puschmann, H., Howard, J., & Low, P. (2001). Electrochemical evidence for electronic interactions through the para-carborane skeleton in the novel tricluster [{Co2C2(SiMe3)(CO)4(dppm)}2(μ-CB10H10C)]. Chemical Communications, 2001(17), 1610-1611. https://doi.org/10.1039/b104307m
- Synthesis of isomeric B-methylated tantalum carboranes, (Me₂N)₃TaC₂B₉H₁₀MeFox, M., Howard, J., Hughes, A., Malget, J., & Yufit, D. (2001). Synthesis of isomeric B-methylated tantalum carboranes, (Me₂N)₃TaC₂B₉H₁₀Me. Dalton Transactions, 2001(15), 2263-2269. https://doi.org/10.1039/b103353k
- Phosphine promoted substituent redistribution reactions of B- chlorocatechol borane: Molecular structures of ClBcat, BrBcat and L center dot ClBcat (cat=1,2-O2C6H4; L = PMe3, PEt3, PBu3t, PCy3, NEt3)Coapes, R. B., Souza, F. E. S., Fox, M. A., Batsanov, A. S., Goeta, A. E., Yufit, D. S., Leech, M., Howard, J. A. K., Scott, A. J., Clegg, W., & Marder, T. B. (2001). Phosphine promoted substituent redistribution reactions of B- chlorocatechol borane: Molecular structures of ClBcat, BrBcat and L center dot ClBcat (cat=1,2-O2C6H4; L = PMe3, PEt3, PBu3t, PCy3, NEt3). Dalton Transactions., 2001(8), 1201-1209. https://doi.org/10.1039/B010025K
- Empirical and ab initio energy/architectural patterns for 73 nido-6 < V >-carborane isomers, from B6H9- to C4B2H6Fox, M. (2001). Empirical and ab initio energy/architectural patterns for 73 nido-6 < V >-carborane isomers, from B6H9- to C4B2H6. Inorganic Chemistry, 40(8), 1790-1801.
- The molecular structure of (PSH+)(nido-7,8-C2B9H12-) determined by neutron diffraction (PS = proton sponge, 1,8- bis(dimethylamino)naphthalene)Fox, M., Goeta, A., Howard, J., Hughes, A., Johnson, A., Keen, D., Wade, K., & Wilson, C. (2001). The molecular structure of (PSH+)(nido-7,8-C2B9H12-) determined by neutron diffraction (PS = proton sponge, 1,8- bis(dimethylamino)naphthalene). Inorganic Chemistry, 40(1), 173-+.
- Why are B2O2 rings rare?Burke, J. M., Fox, M. A., Goeta, A. E., Hughes, A. K., & Marder, T. B. (2000). Why are B2O2 rings rare?. Chemical Communications, 2000(22), 2217-2218. https://doi.org/10.1039/B006685K
- First structural characterisation of a 2,1,12-MC2B9 metallacarborane, [2,2,2-(NMe2)(3)-closo-2,1,12-TaC2B9H11]. Trends in boron NMR shifts on replacing a BH vertex with a metal MLn vertex in icosahedral carboranesBatsanov, A. S., Eva, P. A., Fox, M. A., Howard, J. A. K., Hughes, A. K., Johnson, A. L., Martin, A. M., & Wade, K. (2000). First structural characterisation of a 2,1,12-MC2B9 metallacarborane, [2,2,2-(NMe2)(3)-closo-2,1,12-TaC2B9H11]. Trends in boron NMR shifts on replacing a BH vertex with a metal MLn vertex in icosahedral carboranes. Dalton Transactions., 2000(20), 3519-3525. https://doi.org/10.1039/B005294I
- 1,2-Ph2-9-1-1,2-closo-C2B10H9 - Steric effects in heteroboranes. Part 24Fox, M. (2000). 1,2-Ph2-9-1-1,2-closo-C2B10H9 - Steric effects in heteroboranes. Part 24. Acta Crystallographica C: Crystal Structure Communications, 56, 487-488. https://doi.org/10.1107/s0108270100000743
- Synthesis and structure of 1,12-diethynyl-para-carboraneFox, M. (2000). Synthesis and structure of 1,12-diethynyl-para-carborane. Journal of Organometallic Chemistry, 610(1-2), 20-24.
- Syntheses and reactions of some new C-pentafluorophenyl and tetrafluorophenylene carborane systemsFox, M. (2000). Syntheses and reactions of some new C-pentafluorophenyl and tetrafluorophenylene carborane systems. Journal of Organometallic Chemistry, 597(1-2), 157-163.
- Gas-phase reactions of nido-1-methylpentaborane with propyne and 2-pentyne. Formation of B-alkyl nido and close carbaboranesFox, M. (2000). Gas-phase reactions of nido-1-methylpentaborane with propyne and 2-pentyne. Formation of B-alkyl nido and close carbaboranes. Journal of Organometallic Chemistry, 614, 262-268.
- Deboronation of 9-substituted-ortho- and -meta-carboranesFox, M. (1999). Deboronation of 9-substituted-ortho- and -meta-carboranes. Journal of Organometallic Chemistry, 573(1-2), 279-291.
- Deboronation of ortho-carborane by an iminophosphorane: crystal structures of the novel carborane adduct nido-C2B10H12 center dot HNP(NMe2)(3) and the borenium salt [(Me2N)(3)PNHBNP(NMe2)(3)](2)O2+(C2B9H12-)(2)Davidson, M. G., Fox, M. A., Hibbert, T. G., Howard, J. A. .K., Mackinnon, A., Neretin, I. S., & Wade, K. (1999). Deboronation of ortho-carborane by an iminophosphorane: crystal structures of the novel carborane adduct nido-C2B10H12 center dot HNP(NMe2)(3) and the borenium salt [(Me2N)(3)PNHBNP(NMe2)(3)](2)O2+(C2B9H12-)(2). Chemical Communications, 1999(17), 1649-1650. https://doi.org/10.1039/A903030A
- Gas-phase flash reactions of diborane, triborane carbonyl and tetraborane with alkynesFox, M. (1999). Gas-phase flash reactions of diborane, triborane carbonyl and tetraborane with alkynes. Collection of Czechoslovak Chemical Communications., 64(5), 806-818. https://doi.org/10.1135/cccc19990806
- Six-vertex nido-carborane structures with unusual CHB bridges or endo-CH hydrogensFox, M. (1998). Six-vertex nido-carborane structures with unusual CHB bridges or endo-CH hydrogens. Journal of Organometallic Chemistry, 550(1-2), 331-340.
- Molecular structures of 1,12-B12H10(CO)(2) and its dihydrate 1,12-B12H10[C(OH)(2)](2) - a novel bis-carbene complexFox, M. A., Howard, J. A. K., Moloney, J. M., & Wade, K. (1998). Molecular structures of 1,12-B12H10(CO)(2) and its dihydrate 1,12-B12H10[C(OH)(2)](2) - a novel bis-carbene complex. Chemical Communications, 1998(22), 2487-2488. https://doi.org/10.1039/A806898D
- Studies on tetraborane(8) carbonyl, B4H8.CO: its isomeric composition in the gas phase and in solution, and its reactions with alkenesFox, M. (1998). Studies on tetraborane(8) carbonyl, B4H8.CO: its isomeric composition in the gas phase and in solution, and its reactions with alkenes. Journal of Organometallic Chemistry, 550(1-2), 207-212.
- Transmission of electronic effects by icosahedral carboranes: skeletal carbon-13 chemical shifts and ultraviolet-visible spectra of substituted aryl-p-carboranes (1,12-dicarba-closo-dodecaboranes)Wade, K., Fox, M., McBride, J., & Peace, R. (1998). Transmission of electronic effects by icosahedral carboranes: skeletal carbon-13 chemical shifts and ultraviolet-visible spectra of substituted aryl-p-carboranes (1,12-dicarba-closo-dodecaboranes). Dalton Transactions, 1998(3), 401-411.
- Gas-phase reaction of tetraborane(10) and ethyne: Molecular structure of nido-1,2-C2B3H7 in the gas phaseFox, M. (1998). Gas-phase reaction of tetraborane(10) and ethyne: Molecular structure of nido-1,2-C2B3H7 in the gas phase. Inorganic Chemistry, 37(9), 2166-2176.
- 2,4-Ethanotetraborane derivatives. 3. Determination of the molecular structure of 2,4-(t-butylethano)tetraborane(10), 2,4-((BuCHCH2)-C-t)B4H8, in the gas phase by electron diffractionFox, M. (1998). 2,4-Ethanotetraborane derivatives. 3. Determination of the molecular structure of 2,4-(t-butylethano)tetraborane(10), 2,4-((BuCHCH2)-C-t)B4H8, in the gas phase by electron diffraction. Journal of Molecular Structure, 445(1-3), 319-334.
- Cage-fluorination during deboronation of meta-carboranesFox, M. (1997). Cage-fluorination during deboronation of meta-carboranes. Polyhedron, 16(14), 2517-2525.
- Fluoride-ion deboronation of p-fluorophenyl-ortho- and -meta-carboranes. NMR evidence for the new fluoroborate, HOBHF2-Fox, M. (1997). Fluoride-ion deboronation of p-fluorophenyl-ortho- and -meta-carboranes. NMR evidence for the new fluoroborate, HOBHF2-. Polyhedron, 16(14), 2499-2507.
- Convenient direct syntheses of novel fused-ring CB4N5 systems by nitrile hydroborationCoult, R., Fox, M. A., Rand, B., Wade, K., & Westwood, A. V. K. (1997). Convenient direct syntheses of novel fused-ring CB4N5 systems by nitrile hydroboration. Dalton Transactions., 1997(19), 3411-3413. https://doi.org/10.1039/A705523D
- Molecular structure of trifluorophosphine tetraborane(8), B4H8PF3, as determined in the gas phase by electron diffraction and ab initio computationsFox, M. (1997). Molecular structure of trifluorophosphine tetraborane(8), B4H8PF3, as determined in the gas phase by electron diffraction and ab initio computations. Inorganic Chemistry, 36(6), 1048-1054.
- Gas-phase reaction of tetrahorane(10) with allene: The fluxional arachno-1-carbapentaborane(10) isomeric system and derivatives 1,2- and 1,3-Me-2-1-CB4H8; analogies in 1-CB4H10, MeB5H10, and B5H10-Fox, M. (1997). Gas-phase reaction of tetrahorane(10) with allene: The fluxional arachno-1-carbapentaborane(10) isomeric system and derivatives 1,2- and 1,3-Me-2-1-CB4H8; analogies in 1-CB4H10, MeB5H10, and B5H10-. Angewandte Chemie International Edition, 36(13-14), 1498-1501. https://doi.org/10.1002/anie.199714981
- Some boron-containing ring systemsFox, M. (1997). Some boron-containing ring systems. Phosphorus, Sulfur, and Silicon and the Related Elements, 125, 73-82.
- Crystallographic evidence for the diene character of C2B10H10C4H4 ('benzocarborane') and a Diels-Alder reaction of its anionic nido-analogue, [C2B9H10C4H4](-): Crystal structures of C2B10H10C4H4 and C2B10H10C4H6Copley, R. C. B., Fox, M. A., Gill, W. R., Howard, J. A. K., MacBride, J. A. H., Peace, R. J., Rivers, G. P., & Wade, K. (1996). Crystallographic evidence for the diene character of C2B10H10C4H4 (’benzocarborane’) and a Diels-Alder reaction of its anionic nido-analogue, [C2B9H10C4H4](-): Crystal structures of C2B10H10C4H4 and C2B10H10C4H6. Chemical Communications, 1996(17), 2033-2034. https://doi.org/10.1039/CC9960002033
- Gas-phase reactions of tetraborane(10) with 1-en-3-ynes: Syntheses of the parent tricarbahexaborane, nido-2,3,4-C3B3H7, and its derivativesFox, M. A., Greatrex, R., & Nikrahi, A. (1996). Gas-phase reactions of tetraborane(10) with 1-en-3-ynes: Syntheses of the parent tricarbahexaborane, nido-2,3,4-C3B3H7, and its derivatives. Chemical Communications, 1996(2), 175-176. https://doi.org/10.1039/CC9960000175
- Existence of C,3-Me(2)-closo-1,2-C2B3H3 refuted by the ab initio IGLO, GIAO-MP2/NMR method. Attempted repetition of the synthesisFox, M. (1996). Existence of C,3-Me(2)-closo-1,2-C2B3H3 refuted by the ab initio IGLO, GIAO-MP2/NMR method. Attempted repetition of the synthesis. Inorganic Chemistry, 35(21), 6170-6178.
- Deboronation of C-substituted ortho- and meta-closo-carboranes using ''wet'' fluoride ion solutionsFox, M. (1996). Deboronation of C-substituted ortho- and meta-closo-carboranes using ’’wet’’ fluoride ion solutions. Polyhedron, 15(4), 565-571.
- Re-identification of the major volatile carbaboranes from the gas-phase reactions of tetraborane(10) and alkynes at 50 °CFox, M. A., & Greatrex, R. (1995). Re-identification of the major volatile carbaboranes from the gas-phase reactions of tetraborane(10) and alkynes at 50 °C. Journal of the Chemical Society, Chemical Communications, 1995(6), 667-668. https://doi.org/10.1039/C39950000667
- 2,4-ETHANOTETRABORANE DERIVATIVES .2. SYNTHESIS, CHARACTERIZATION, AND GAS-PHASE STRUCTURES OF 2,4-(MECHCH(2))B4H8, 2,4-(TRANS-MECHCHME)B4H8, AND 2-PR-2,4-(MECHCH(2))B4H7 AND 4-PR-2,4-(MECHCH(2))B4H7Fox, M. (1995). 2,4-ETHANOTETRABORANE DERIVATIVES .2. SYNTHESIS, CHARACTERIZATION, AND GAS-PHASE STRUCTURES OF 2,4-(MECHCH(2))B4H8, 2,4-(TRANS-MECHCHME)B4H8, AND 2-PR-2,4-(MECHCH(2))B4H7 AND 4-PR-2,4-(MECHCH(2))B4H7. Inorganic Chemistry, 34(11), 2841-2849.
- THE STRUCTURES OF ALKYL DERIVATIVES OF ARACHNO-1-CB4H10 FROM REACTIONS OF B4H10 WITH ALKYNESFox, M. (1994). THE STRUCTURES OF ALKYL DERIVATIVES OF ARACHNO-1-CB4H10 FROM REACTIONS OF B4H10 WITH ALKYNES. Angewandte Chemie International Edition, 33(22), 2298-2300. https://doi.org/10.1002/anie.199422981
- The true identity of the ‘bare-carbon’ cluster, closo-C3B5H7Fox, M. A., & Greatrex, R. (1994). The true identity of the ‘bare-carbon’ cluster, closo-C3B5H7. Dalton Transactions., 1994(21), 3197-3198. https://doi.org/10.1039/DT9940003197
- X-RAY STRUCTURE AND BONDING OF 1-PHENYLETHYNYL-2-PHENYL-1,2-DICARBADODECABORANE(12), [1-(PHC=C)-2-PH-1,2-C2B10H10], A MODEL ALKYNE COMPLEX CONTAINING A RICH VARIETY OF CARBON-CARBON BOND TYPESFox, M. (1993). X-RAY STRUCTURE AND BONDING OF 1-PHENYLETHYNYL-2-PHENYL-1,2-DICARBADODECABORANE(12), [1-(PHC=C)-2-PH-1,2-C2B10H10], A MODEL ALKYNE COMPLEX CONTAINING A RICH VARIETY OF CARBON-CARBON BOND TYPES. Polyhedron, 12(22), 2711-2717.
- Even more reliable NMR chemical shift computations by the GIAO-MP2 methodSchleyer, P. von R., Gauss, J., Bühl, M., Greatrex, R., & Fox, M. A. (1993). Even more reliable NMR chemical shift computations by the GIAO-MP2 method. Journal of the Chemical Society, Chemical Communications, 1993(23), 1766-1768. https://doi.org/10.1039/C39930001766
- C-ARYLATION AND C-HETEROARYLATION OF ICOSAHEDRAL CARBORANES VIA THEIR COPPER(I) DERIVATIVESFox, M. (1993). C-ARYLATION AND C-HETEROARYLATION OF ICOSAHEDRAL CARBORANES VIA THEIR COPPER(I) DERIVATIVES. Journal of Organometallic Chemistry, 462(1-2), 19-29.
- PROPOSED STRUCTURE OF THE 1ST HYPHO CARBABORANE, C3B4H12Fox, M. (1993). PROPOSED STRUCTURE OF THE 1ST HYPHO CARBABORANE, C3B4H12. Polyhedron, 12(15), 1849-1853.
- A PENTUPLY-BRIDGING THIOCARBONYL GROUP - X-RAY CRYSTAL-STRUCTURE OF A SALT OF THE 1-THIO-2-PHENYL-1,2-DICARBADODECABORATE(12) ANION, [LH]+[S(PH)C2B10H10]- (L = 1,8-N,N,N1,N1-TETRAMETHYLNAPHTHALENE DIAMINE)Fox, M. (1992). A PENTUPLY-BRIDGING THIOCARBONYL GROUP - X-RAY CRYSTAL-STRUCTURE OF A SALT OF THE 1-THIO-2-PHENYL-1,2-DICARBADODECABORATE(12) ANION, [LH]+[S(PH)C2B10H10]- (L = 1,8-N,N,N1,N1-TETRAMETHYLNAPHTHALENE DIAMINE). Polyhedron, 11(20), 2717-2721.

