Staff profile
Professor Patrick Steel
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42131 |
| Professor in the Biophysical Sciences Institute | |
| Biophysical Sciences Institute Executive Board in the Biophysical Sciences Institute |
Biography
Research Interests
The Steel group has a broad spread of interests ranging from the development and applications of new methods for organic synthesis to the design and synthesis of small molecule probes and modulators (inhibitors) of biological processes to applications in applied synthetic biology. The work is supported by both the research councils (EPSRC, BBSRC, MRC) and industry (Syngenta, GSK, AZ, Pfizer, Lilly)
Synthetic Chemistry
Synthesis and Application of Boronic Acids
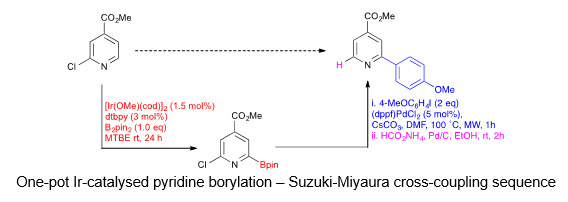
Aryl and heteroaryl boronate esters are very important organic intermediates in organic synthesis that have been deployed in many useful transformations including the Suzuki–Miyaura cross-coupling reactions, Cu-catalyzed C-O and C-N coupling reactions, and conjugate additions to carbonyl compounds. As a result there is a need for efficient methods to prepare them. We have been studying the iridium catalyzed direct borylation of C-H and C-X bonds as a powerful route to these compounds.[1] A particular current interest is in developing strategies for the preparation of basic heterocyclic boronic acid which are more challenging to prepare but are of vital importance for the pharmaceutical and agrochemical industries.[2] Other goals include included the development of sequential chemistries in which borylation is combined with a second transformation,[3] the design of better ligand sets to control regiochemistry and the development of faster, more effective catalysts using cheaper metals such as copper and zinc.[4] Throughout all of this we collaborate closely with Todd Marder (Würzburg) and also with other chemists elsewhere including Profs Zenyang Lin (Hong Kong) and Lei Lui (Beijing).
Organosilicon Chemistry
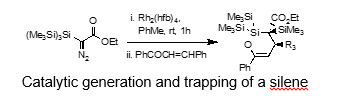
We have a longstanding interest in the chemistry of low-coordination silicon compounds as applied to organic synthesis. Owing to their transient existence, such π-bonded silicon compounds have primarily been the subject of research into fundamental aspects of structure and reactivity with little attempt to explore their potential in organic synthesis.[5] We have been particularly interested in studying the cycloaddition chemistry of silenes (compounds with a silicon carbon double bond). These prove to be powerful reagents for stereocontrolled alkene functionalization and we have used this methodology in the synthesis of novel heterocycles and natural products.[6] A current challenge is to develop methods which provide access to the silene under milder catalytic conditions to increase the functional group tolerance of this approach.[7] Much of this chemistry is undertaken in collaboration with groups Europe notably Prof. Henrik Ottosson at Uppsala University in Sweden.
Chemical Biology
We are engaged in a number of projects that fall under the broad umbrella of chemical biology. In all of these a key goal is an understanding of how biological processes and the interactions between biological target and the small molecule are occurring at a molecular level. Much of this work is undertaken in collaboration in which group member move seamless between chemistry and biology groups learning skill in both disciplines. We have a particular interest in plant and parasite chemical biology.
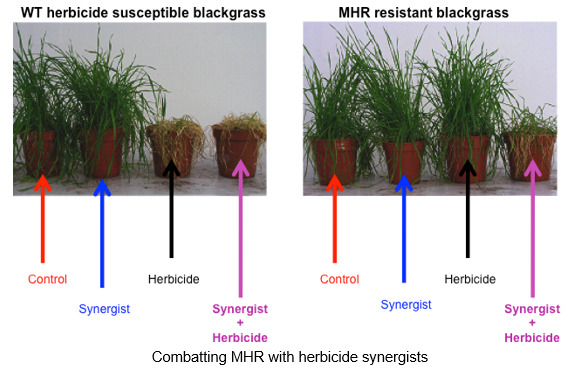
In plant chemical biology we have developed close links with groups working under the umbrella of the Durham Centre for Crop Improvement Technologies (https://www.dur.ac.uk/dccit/). For example, we have collaborated with Prof. Patrick Hussey to generate fluorescent probes to study plant peroxisome dynamics and explore herbicides targeting plant cell wall biosynthesis;[8] with Dr. Ehmke Pohl (Durham), Prof. Robert Edwards (Newcastle University) we are studying enzymes associated with herbicide detoxification and resistance with a specific goal to synthesise new herbicide synergists to understand and overcome multiple herbicide resistance in grass weeds.[9] More recently, with Prof. Mark Knight (Durham) we have started a programme probing the role of calcium signaling in plants particularly in relation to root development under conditions of stress (drought, freezing etc).
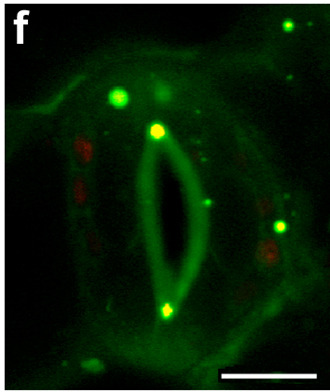
Image showing co-localisation (yellow staining) of peroxisome probe PTS1-mCherry (red) and Durham synthesised BODIPYprobe (green) in Nicotiana benthamiana stomata cells. Scale bar 10 mm.
Our interests in parasite chemical biology focus on leishmaniasis and human African trypanosomiasis (both African Sleeping Sickness and Chagas disease). These are some of the Worlds most serious neglected diseases with over 350 million people considered to be at risk and result in excess of 3.3 million DALYs. We have a major ongoing collaboration with Dr Paul Denny (Durham) supported by both MRCT Ltd and GSK to develop new therapies for leishmaniasis. Towards this end we have identified enzymes associated with complex sphinglipid biosynthesis as potential new drug targets, developed assays and used these to identify compounds with activity and selectivity that we are currently optimising.[10] In parallel, and in collaboration with Prof. Bartira Bergmann (Rio de Janeiro), we are developing probes to identify and validate new drug targets for this disease. Ultimately we hope to find compounds that have the potential to make a difference to some of the world’s poorest people.
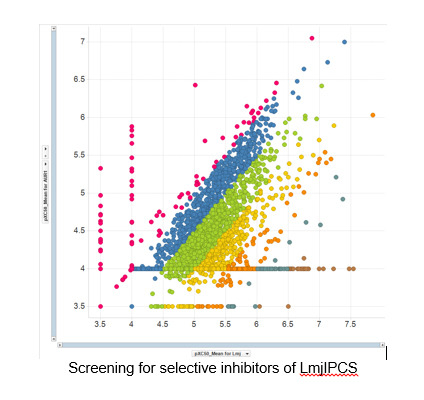
References
[1] C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder, and L. Liu, ‘Alkylboronic Esters from Copper-Catalyzed Borylation of Primary and Secondary Alkyl Halides and Pseudo-Halides’, Angew.Chem. Int. Ed., 2012, 51, 528-532.
[2] H. Tajuddin , P. Harrisson , B. Bitterlich , J. C. Collings , N. Sim , A. S. Batsanov, M. S.Cheung , S. Kawamorita , A. C. Maxwell , L. Shukla , J. Morris , Z. Lin , T. B. Marder and P. G. Steel, ‘Iridium-catalyzed C–H borylation of quinolines and unsymmetrical 1,2- disubstituted benzenes: insights into steric and electronic effects on selectivity’, Chem. Sci., 2012, 3, 3505-3515; S. A. Sadler, H. Tajuddin, I. A. I. Mkhalid, A. S. Batsanov, M. S. Cheung, A. C. Maxwell, L. Shukla, B. Roberts, D. Blakemore, Z. Lin, T. B. Marder and P. G. Steel, ‘Iridium-Catalyzed C-H Borylation of Pyridines’Org. Biomol. Chem. 2014., 12, 7318 – 7327.
[3] P. Harrisson, J. Morris, P. G. Steel and T. B. Marder, ‘A One-Pot, Single-Solvent Process for Tandem, Catalyzed C–H Borylation–Suzuki–Miyaura Cross-Coupling Sequences’, Synlett., 2009, 147-150; H. Tajuddin, L. Shukla, A. Maxwell, T. B. Marder and P. G. Steel, ‘A “One-Pot” Tandem C-H Borylation/1,4-Conjugate Addition/Reduction Sequence’, Org. Lett., 2010, 12, 5700-5703.
[4] S. K. Bose, K. Fucke, L. Liu, P. G. Steel, T. B. Marder ‘Zinc-Catalyzed Borylation of Primary, Secondary and Tertiary Alkyl Halides with Alkoxy Diboron Reagents at Room Temperature’, Angew.Chem. Int. Ed., 2014, 53, 1799-1803
[5] H. Ottosson and P. G. Steel, ‘Silylenes, Silenes, and Disilenes: Novel silicon based reagents for organic synthesis?’ Chem. Eur. J., 2006, 12, 1576-1585.
[6] R. D. C. Pullin, J. D. Sellars, and P. G. Steel, ‘Total Synthesis of (±)-Epipicropodophyllin’ Org. Biomol. Chem., 2007, 5, 3201-3206. J. D. Sellars and P. G. Steel, ‘Application of Silacyclic Allylsilanes to the Synthesis of b-Hydroxy-d-Lactones: Synthesis of Prelactone B‘, Tetrahedron., 2009, 65, 5588-5595. M. Czyzewski, J. D. Sellars, T. Guliashvili, J. Tibbelin, Julius, L. Johnstone, J. Bower, M. Box, R. D. M. Davies, H. Ottosson, and P. G. Steel, ‘The first intramolecular silene Diels-Alder reactions’, Chem. Commun., 2014, 50, 2919-2921.
[7] M. Czyzewski, J. Bower, M. Box, H. Ottosson and P. G. Steel, ‘Silene Equivalents Through the Rhodium Catalysed Reactions of a-Hypersilyl Diazoesters.’ Chem. Sci., 2011, 2, 2367 -2372.
[8] M. Landrum, A. Smertenko, R. Edwards, P. J. Hussey and P. G. Steel, ‘BODIPY Probes to Study Peroxisome Function in Vivo’ Plant. J., 2010, 62, 529-538.
[9] I. Cummins, D. J. Wortley, F. Sabbadin, Z. He, C. R. Coxon, H. E. Straker, J. Sellars, K. M. Knight, L. Edwards, D. Hughes, S. S. Kaundun, S.-J. Hutchings, P. G. Steel and R. Edwards’ A key role for a glutathione transferase in multiple herbicide resistance in grass weeds’, Proc. Nat. Acad. Sci., 2013, 110, 5812-5817
[10] J. G. Mina, S. Y. Pan, N. K. Wansadhipathi, C. R. Bruce, H. Shams-Eldin, R. T. Schwarz, P. G. Steel, and P. W. Denny, ‘The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug target’ Mol. Biochem. Parasitol., 2009, 168, 16-23; J. G. Mina, J. A. Mosely, H. Shams-Eldin, R. T. Schwarz, P. G. Steel and P. W. Denny, ‘A plate-based assay system for analyses and screening of the Leishmania major inositol phosphorylceramide synthase.’ Int. J. Biochem. Cell. Biol., 2010, 42, 1553-1561; J. G. Mina, J. A. Mosely, H. Z. Ali, P. W. Denny and P. G. Steel, ‘Exploring Leishmania major inositol phosphorylceramide synthase (LmjIPCS): Insights into the ceramide binding domain.’ Org. Biomol. Chem. 2011, 9, 1823; J.L. Norcliffe, E. Alvarez-Ruiz, J.J. Martin-Plaza, P.G. Steel and P.W. Denny, The utility of yeast as a tool for cell-based, target-directed high throughput screening, Parasitology, 2013, 141, 8-16.
Research interests
- Synthetic Organic Chemistry
- Organosilicon Chemistry
- Chemical Biology
Publications
Journal Article
- Profiling Serine Hydrolases in the Leishmania Host‐Pathogen Interactome Using Cell‐Permeable Activity‐Based Fluorophosphonate ProbesIsern, J. A., Porta, E. O., Kalesh, K., Koutsogiannis, Z., Cazzola, D., Pohl, E., Denny, P., & Steel, P. G. (2025). Profiling Serine Hydrolases in the Leishmania Host‐Pathogen Interactome Using Cell‐Permeable Activity‐Based Fluorophosphonate Probes. ChemBioChem, 26(10), Article e202500160. https://doi.org/10.1002/cbic.202500160
- Simple accessible clemastine fumarate analogues as effective antileishmanialsCharlton, R., Escrivani, D., Brown, C., Thota, N., de Sousa Agostino, V., Porta, E., Avkiran, T., Merritt, A. T., Denny, P. W., Rossi-Bergmann, B., & Steel, P. G. (2025). Simple accessible clemastine fumarate analogues as effective antileishmanials. RSC Medicinal Chemistry, 16(4), 1686-1694. https://doi.org/10.1039/d4md01004c
- Nanocrystallization Effectively Improves the Oral Efficacy of an Antileishmanial ChalconeBorsodi, M. P. G., Pacienza-Lima, W., Menezes, J. C. V., Escrivani-Oliveira, D., Arruda-Costa, N., Silva, A. J. M. da, Cabral, L. M., Steel, P. G., Sousa-Batista, A. de J., & Rossi-Bergmann, B. (2025). Nanocrystallization Effectively Improves the Oral Efficacy of an Antileishmanial Chalcone. Pharmaceutics, 17(4), Article 399. https://doi.org/10.3390/pharmaceutics17040399
- Applications of Transition Metal-Catalyzed ortho-Fluorine-Directed C–H Functionalization of (Poly)fluoroarenes in Organic SynthesisBudiman, Y. P., Perutz, R. N., Steel, P. G., Radius, U., & Marder, T. B. (2024). Applications of Transition Metal-Catalyzed ortho-Fluorine-Directed C–H Functionalization of (Poly)fluoroarenes in Organic Synthesis. Chemical Reviews, 124(8), 4822-4862. https://doi.org/10.1021/acs.chemrev.3c00793
- Assessing Dose-Exposure-Response Relationships of Miltefosine in Adults and Children using Physiologically-Based Pharmacokinetic Modeling Approach.Madu, S. J., Wang, K., Chirumamilla, S. K., Turner, D. B., Steel, P. G., & Li, M. (2023). Assessing Dose-Exposure-Response Relationships of Miltefosine in Adults and Children using Physiologically-Based Pharmacokinetic Modeling Approach. Pharmaceutical Research, 40, 2983-3000. https://doi.org/10.1007/s11095-023-03610-0
- Synthesis and vectorial functionalisation of pyrazolo[3,4- c ]pyridinesBedwell, E. V., da Silva Emery, F., Clososki, G. C., & Steel, P. G. (2023). Synthesis and vectorial functionalisation of pyrazolo[3,4- c ]pyridines. RSC Advances, 13(49), 34391-34399. https://doi.org/10.1039/d3ra07458g
- Inhibition of HSP90 distinctively modulates the global phosphoproteome of Leishmania mexicana developmental stagesPorta, E. O., Gao, L., Denny, P. W., Steel, P. G., & Kalesh, K. (2023). Inhibition of HSP90 distinctively modulates the global phosphoproteome of Leishmania mexicana developmental stages. Microbiology Spectrum, 11(6), Article e02960-23. https://doi.org/10.1128/spectrum.02960-23
- Disruption of the inositol phosphorylceramide synthase gene affects Trypanosoma cruzi differentiation and infection capacityDos Santos, N. S. A., Estevez-Castro, C. F., Macedo, J. P., Chame, D. F., Castro-Gomes, T., Santos-Cardoso, M., Burle-Caldas, G. A., Covington, C. N., Steel, P. G., Smith, T. K., Denny, P. W., & Teixeira, S. M. R. (2023). Disruption of the inositol phosphorylceramide synthase gene affects Trypanosoma cruzi differentiation and infection capacity. PLOS Neglected Tropical Diseases, 17(9), Article e0011646. https://doi.org/10.1371/journal.pntd.0011646
- Navigating drug repurposing for Chagas disease: advances, challenges, and opportunitiesPorta, E. O. J., Kalesh, K., & Steel, P. G. (2023). Navigating drug repurposing for Chagas disease: advances, challenges, and opportunities. Frontiers in Pharmacology, 14(2023). https://doi.org/10.3389/fphar.2023.1233253
- Iridium‐Catalysed C−H Borylation of Fluoroarenes: Insights into the Balance between Steric and Electronic Control of RegioselectivityDing, M., Reuven, J. A., Hones, A. C., Fox, M. A., & Steel, P. G. (2022). Iridium‐Catalysed C−H Borylation of Fluoroarenes: Insights into the Balance between Steric and Electronic Control of Regioselectivity. European Journal of Organic Chemistry, 2022(47). https://doi.org/10.1002/ejoc.202201005
- Integral Role of Water in the Solid-State Behavior of the Antileishmanial Drug MiltefosineHall, A. V., Gostick, I. E., Yufit, D. S., Marchant, G. Y., Kirubakaran, P., Madu, S. J., Li, M., Steel, P. G., & Steed, J. W. (2022). Integral Role of Water in the Solid-State Behavior of the Antileishmanial Drug Miltefosine. Crystal Growth and Design, 22(10), 6262-6266. https://doi.org/10.1021/acs.cgd.2c00843
- Plant growth promotion by the interaction of a novel synthetic small molecule with GA‐DELLA functionSukiran, N. A., Pollastri, S., Steel, P. G., & Knight, M. R. (2022). Plant growth promotion by the interaction of a novel synthetic small molecule with GA‐DELLA function. Plant Direct, 6(4), Article e398. https://doi.org/10.1002/pld3.398
- Discovery of Leishmania Druggable Serine Proteases by Activity-Based Protein ProfilingPorta, E. O., Isern, J. A., Kalesh, K., & Steel, P. G. (2022). Discovery of Leishmania Druggable Serine Proteases by Activity-Based Protein Profiling. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.929493
- Flavonoid-based inhibitors of the Phi-class glutathione transferase from black-grass to combat multiple herbicide resistanceSchwarz, M., Eno, R. F., Freitag-Pohl, S., Coxon, C. R., Straker, H. E., Wortley, D. J., Hughes, D. J., Mitchell, G., Moore, J., Cummins, I., Onkokesung, N., Brazier-Hicks, M., Edwards, R., Pohl, E., & Steel, P. G. (2021). Flavonoid-based inhibitors of the Phi-class glutathione transferase from black-grass to combat multiple herbicide resistance. Organic & Biomolecular Chemistry, 19(42), 9211-9222. https://doi.org/10.1039/d1ob01802g
- Iridium Catalysed C-H Borylation of Heteroarenes: Balancing steric and electronic regiocontrolWright, J. S., Scott, P. J., & Steel, P. G. (2021). Iridium Catalysed C-H Borylation of Heteroarenes: Balancing steric and electronic regiocontrol. Angewandte Chemie International Edition, 60(6), 2796-2821. https://doi.org/10.1002/anie.202001520
- Antileishmanial Chemotherapy through Clemastine Fumarate Mediated Inhibition of the Leishmania Inositol Phosphorylceramide SynthaseMina, J. G., Charlton, R. L., Alpizar-Sosa, E., Escrivani, D. O., Brown, C., Alqaisi, A., Borsodi, M. P. G., Figueiredo, C. P., de Lima, E. V., Dickie, E. A., Wei, W., Coutinho-Silva, R., Merritt, A., Smith, T. K., Barrett, M. P., Rossi-Bergmann, B., Denny, P. W., & Steel, P. G. (2021). Antileishmanial Chemotherapy through Clemastine Fumarate Mediated Inhibition of the Leishmania Inositol Phosphorylceramide Synthase. ACS Infectious Diseases, 7(1), 47-63. https://doi.org/10.1021/acsinfecdis.0c00546
- Single-dose treatment for cutaneous leishmaniasis with an easily synthesized chalcone entrapped in polymeric microparticlesSousa-Batista, A. J., Arruda-Costa, N., Escrivani, D. O., Reynaud, F., Steel, P. G., & Rossi-Bergmann, B. (2020). Single-dose treatment for cutaneous leishmaniasis with an easily synthesized chalcone entrapped in polymeric microparticles. Parasitology, 147(9), 1032-1037. https://doi.org/10.1017/s0031182020000712
- Basal stomatal aperture is regulated by GA-DELLAs in ArabidopsisSukiran, N. A., Steel, P. G., & Knight, M. R. (2020). Basal stomatal aperture is regulated by GA-DELLAs in Arabidopsis. Journal of Plant Physiology, 250, Article 153182. https://doi.org/10.1016/j.jplph.2020.153182
- Encapsulation in lipid-core nanocapsules improves topical treatment with the potent antileishmanial compound CH8Escrivani, D. O., Lopes, M. V., Poletto, F., Ferrarini, S. R., Sousa-Batista, A. J., Steel, P. G., Guterres, S. S., Pohlmann, A. R., & Rossi-Bergmann, B. (2020). Encapsulation in lipid-core nanocapsules improves topical treatment with the potent antileishmanial compound CH8. Nanomedicine: Nanotechnology, Biology and Medicine, 24, Article 102121. https://doi.org/10.1016/j.nano.2019.102121
- Exploiting C-H Borylation for the Multidirectional Elaboration of 2-HalopyridinesReuven, J., Salih, O., Sadler, S., Thomas, C., & Steel, P. (2020). Exploiting C-H Borylation for the Multidirectional Elaboration of 2-Halopyridines. Tetrahedron, 76(3), Article 130836. https://doi.org/10.1016/j.tet.2019.130836
- C-H activation/metalation approaches for the synthesis of indolizine derivativesClososki, G. C., Steel, P. G., Bertallo, C. R. de S., Arroio, T. R. A. R., Vessecchi, R., Toledo, M. F., & Sadler, S. (2019). C-H activation/metalation approaches for the synthesis of indolizine derivatives. European Journal of Organic Chemistry, 31-32, 5205-5213. https://doi.org/10.1002/ejoc.201900608
- Impact of heat and drought stress on peroxisome proliferation in quinoaHinojosa, L., Sanad, M., Jarvis, D., Steel, P., Murphy, K., & Smertenko, A. (2019). Impact of heat and drought stress on peroxisome proliferation in quinoa. Plant Journal, 99(6), 1144-1158. https://doi.org/10.1111/tpj.14411
- Identification of Novel Benzoxa-[2,1,3]-diazole Substituted Amino Acid Hydrazides as Potential Anti-Tubercular AgentsBrown, A., Aljohani, A., Gill, J., Steel, P., & Sellars, J. (2019). Identification of Novel Benzoxa-[2,1,3]-diazole Substituted Amino Acid Hydrazides as Potential Anti-Tubercular Agents. Molecules, 24(4), Article 811. https://doi.org/10.3390/molecules24040811
- Identifying inhibitors of the Leishmania inositol phosphorylceramide synthase with antiprotozoal activity using a yeast-based assay and ultra-high throughput screening platformNorcliffe, J., Mina, J., Alvarez-Ruiz, E., Cantizani-Perez, J., de Dios-Anton, F., Colmenarejo, G., Gonzalez-Del Valle, S., Marco-Martin, M., Fiandor, J., Martin-Plaza, J., Steel, P., & Denny, P. (2018). Identifying inhibitors of the Leishmania inositol phosphorylceramide synthase with antiprotozoal activity using a yeast-based assay and ultra-high throughput screening platform. Scientific Reports, 8, Article 3938. https://doi.org/10.1038/s41598-018-22063-9
- Repurposing as a Strategy for the Discovery of New Anti-Leishmanials: The-State-of-the-ArtCharlton, R., Rossi-Bergmann, B., Denny, P. W., & Steel, P. G. (2018). Repurposing as a Strategy for the Discovery of New Anti-Leishmanials: The-State-of-the-Art. Parasitology, 145(2), 219-236. https://doi.org/10.1017/s0031182017000993
- Impact of salt stress, cell death, and autophagy on peroxisomes: quantitative and morphological analyses using small Fluorescent probe N-BODIPYFahy, D., Sanad, M. N., Duscha, K., Lyons, M., Liu, F., Bozhkov, P., Kunz, H., Hu, J., Neuhaus, H. E., Steel, P. G., & Smertenko, A. (2017). Impact of salt stress, cell death, and autophagy on peroxisomes: quantitative and morphological analyses using small Fluorescent probe N-BODIPY. Scientific Reports, 7, Article 39069. https://doi.org/10.1038/srep39069
- A mild copper catalyzed method for the selective deprotection of aryl allyl ethersHemming, D. S., Talbot, E. P., & Steel, P. G. (2017). A mild copper catalyzed method for the selective deprotection of aryl allyl ethers. Tetrahedron Letters, 58(1), 17-20. https://doi.org/10.1016/j.tetlet.2016.11.084
- Zinc-Catalyzed Dual C–X and C–H Borylation of Aryl HalidesBose, S. K., Deißenberger, A., Eichhorn, A., Steel, P. G., Lin, Z., & Marder, T. B. (2015). Zinc-Catalyzed Dual C–X and C–H Borylation of Aryl Halides. Angewandte Chemie International Edition, 54(40), 11843-11847. https://doi.org/10.1002/anie.201505603
- Multidirectional Synthesis of Substituted Indazoles via Iridium-Catalyzed C-H BorylationSadler, S., Hones, A., Roberts, B., Blakemore, D., Marder, T., & Steel, P. (2015). Multidirectional Synthesis of Substituted Indazoles via Iridium-Catalyzed C-H Borylation. Journal of Organic Chemistry, 80(10), 5308-5314. https://doi.org/10.1021/acs.joc.5b00452
- Iridium-catalyzed C–H borylation of pyridinesSadler, S., Tajuddin, H., Mkhalid, I., Batsanov, A., Albesa-Jove, D., Cheung, M., Maxwell, A., Shukla, L., Roberts, B., Blakemore, D., Lin, Z., Marder, T., & Steel, P. (2014). Iridium-catalyzed C–H borylation of pyridines. Organic and Biomolecular Chemistry, 12(37), 7318-7327. https://doi.org/10.1039/c4ob01565g
- The First Intramolecular Silene Diels-Alder ReactionsCzyzewski, M., Sellars, J., Guliashvili, T., Tibbelin, J., Johnstone, L., Bower, J., Matthew, B., Davies, R., Henrik Ottosson, H., & Steel, P. (2014). The First Intramolecular Silene Diels-Alder Reactions. Chemical Communications, 50(22), 2919-2921. https://doi.org/10.1039/c3cc49526d
- Iridium-catalyzed C–H borylation of quinolines and unsymmetrical 1,2-disubstituted benzenes: insights into steric and electronic effects on selectivityTajuddin, H., Harrisson, P., Bitterlich, B., Collings, J., Sim, N., Batsanov, A., Cheung, M., Kawamorita, S., Maxwell, A., Shukla, L., Morris, J., Lin, Z., Marder, T., & Steel, P. (2012). Iridium-catalyzed C–H borylation of quinolines and unsymmetrical 1,2-disubstituted benzenes: insights into steric and electronic effects on selectivity. Chemical Science, 3(12), 3505-3515. https://doi.org/10.1039/c2sc20776a
- The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug targetMina, J., Pan, S., Wansadhipathi, N., Bruce, C., Shams-Eldin, H., Schwarz, R., Steel, P., & Denny, P. (2009). The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug target. Molecular and Biochemical Parasitology, 168(1), 16-23. https://doi.org/10.1016/j.molbiopara.2009.06.002
- Hosomi-Sakurai Reactions of SilacyclohexenesHughes, N., Pullin, R., Sanganee, M., Sellars, J., Steel, P., & Turner, M. (2007). Hosomi-Sakurai Reactions of Silacyclohexenes. Organic and Biomolecular Chemistry, 5(17), 2841-2848. https://doi.org/10.1039/b709318g
- The dihydrofuran template approach to furofuran synthesisAldous, D., Batsanov, A., Yufit, D., Dalencon, A., Dutton, W., & Steel, P. (2006). The dihydrofuran template approach to furofuran synthesis. Organic and Biomolecular Chemistry, 4(15), 2912-2927. https://doi.org/10.1039/b604952d
- Silenes in organic synthesis: a short synthesis of prelactone BSellars, J., & Steel, P. (2006). Silenes in organic synthesis: a short synthesis of prelactone B. Organic and Biomolecular Chemistry, 4(17), 3223-3224. https://doi.org/10.1039/b608989e
- Unique Regulation of the Active site of the Serine Esterase S-Formylglutathione Hydrolase.Cummins, I., McAuley, K., Fordham-Skelton, A., Schwoerer, R., Steel, P. G., Davis, B. G., & Edwards, R. (2006). Unique Regulation of the Active site of the Serine Esterase S-Formylglutathione Hydrolase. Journal of Molecular Biology, 359(2), 422-432. https://doi.org/10.1016/j.jmb.2006.03.048
- Diversity linker units for solid-phase organic synthesis.Scott, P., & Steel, P. (2006). Diversity linker units for solid-phase organic synthesis. European Journal of Organic Chemistry, 2006(10), 2251-2268. https://doi.org/10.1002/ejoc.200500804
- Hosomi-Sakurai reactions of silacyclic allyl silanesSellars, J., Steel, P., & Turner, M. (2006). Hosomi-Sakurai reactions of silacyclic allyl silanes. Chemical Communications, 2006(22), 2385-2387. https://doi.org/10.1039/b602642g
- The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acidMasesane, I., Batsanov, A., Howard, J., Mondal, R., & Steel, P. (2006). The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acid. Beilstein Journal of Organic Chemistry, 2(9), Article 9. https://doi.org/10.1186/1860-5397-2-9
Patent
- TREATMENT OF LEISHMANIASISDenny, P., Steel, P., & Lyne, V. (2024). TREATMENT OF LEISHMANIASIS (Patent No. WO 2024/084186 A1).
Working Paper
- Activity-Based Protein Profiling: A Graphical ReviewPorta, E. O. J., & Steel, P. G. (2023). Activity-Based Protein Profiling: A Graphical Review. SSRN. https://doi.org/10.2139/ssrn.4435374

