Staff profile
Professor Philip Dyer
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42150 |
Biography
Research Interests
The group's research broadly spans the areas of synthetic inorganic chemistry, organometallic, organophosphorus and co-ordination chemistry with much of their work focusing on the preparation and characterisation of molecular transition metal complexes and functionalised organic and heteroatom-containing ligands. These interests are driven by the potential application of these species in industrially-relevant small molecule catalysis. On-going targets include the development of new initiator systems for olefin oligomerisation, co-oligomerisation, and polymerisation; gas-to-liquids technology (GTL); carbonylation; CO2 activation; and hydroformylation. In parallel, the group are exploiting the unusual steric and electronic demands of main group fragments (especially those containing phosphorus) for the stabilisation of a range of unusual, often highly reactive molecular species, which they are starting to use as building blocks for the preparation of tuneable, extended pi-conjugated systems and as "responsive ligands". The group is a part of the Department's Centre for Sustainable Process Chemistry (CSCP).
Further information and research details may be found on the group's web pages.
Selected Research Highlights
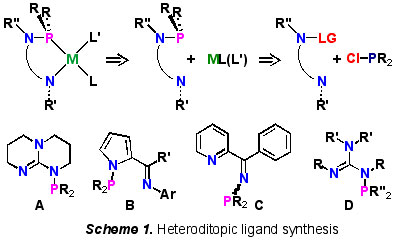
1) Multi-functional Heteroditopic Chelate Ligands
Straightforward phosphorus-nitrogen bond-forming reactions provide ready access to a diverse array of multifunctional, heteroditopic P,N-chelate ligands (Scheme 1). P,N-Compounds A-C have found application as ligands for metals in selective alkene dimerisation [2], oligomerisation [3], and alkoxy-carbonylation [4], respectively, while N-phosphino-guanidine D exhibits novel phosphotropic behaviour [5].
2) “Non-Innocent” Organophosphorus Compounds
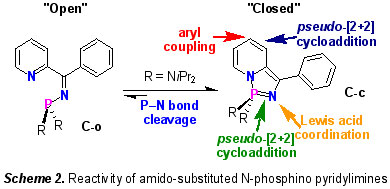
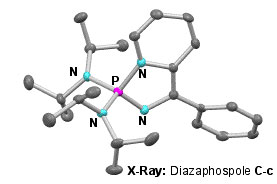
The concept of "non-innocent ligands" (NILs) is well-established in transition metal coordination chemistry and describes metal scaffolds that are subject to redox changes at a site remote from the metal. In contrast, there has been little study of redox active motifs at the periphery of main group elements. We have shown that, depending on the nature of the substituents at the phosphorus centre of the N-phosphino-pyridyl imines C, it is possible to exploit the redox behaviour of the pyridyl imine moiety to set up an equilibrium between the 'open' C-o and 'closed' C-c tautomers (Scheme 2) [6]. This equilibrium mixture exhibits a diverse array of chemistry, which can be further 'tuned' by varying the electrophilicity of the phosphorus substituents (R = amido, alkoxy, etc.).
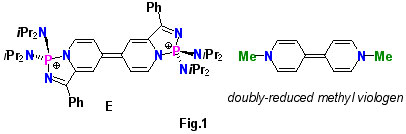
This chemistry opens up an unusual Scholl-type aryl coupling methodology for heterocycles C-c, which may be used to prepare air- and water-stable diphosphonium salts E (Fig. 1). These compounds are analogues of widely studied, electroactive, doubly-reduced viologens, but with a significantly extended pi-conjugated framework, exhibiting interesting photophysical behaviour [7].
3) “Responsive” Phosphine-based Ligands
a) Variable-coordination Scaffolds
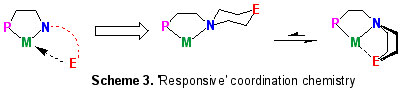
We have started to exploit geometric constraints imposed by organic ligand scaffolds to provide a "flexible" on/off ligand 'arm' binding in conjunction with an asymmetric coordination environment, something of relevance in balancing the steric and electronic demands of many metal-based catalytic cycles (Scheme 3) [8].
b) Redox-driven Coordination Flexibility
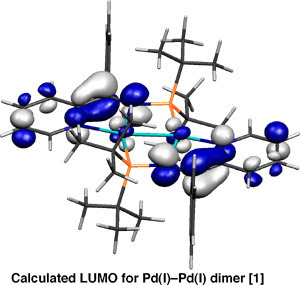
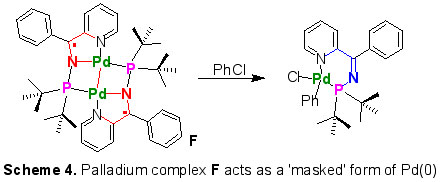
Building on the intriguing and versatile chemistry surrounding pyridyl phosphino imines C, we have begun to explore their use as ligands. Compound C behaves as an unusual multidentate ligand in the coordination sphere of palladium. The neutral form of C coordinates as a kappa-2-P,N-pyridyl ligand [4], while in its mono-reduced form it behaves as anionic kappa-3-P,N,N scaffold bearing a localised radical, e.g. F. Notably, the Pd(I)-Pd(I) dimer F behaves as a 'masked' form of Pd(0) in its reactions (Scheme 5) [1].
4) Tungsten-initiated Selective Alkene Dimerisation
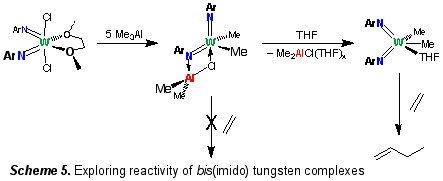
Selective alkene dimerisation is an important industrial process used in the manufacture of plastics, fuels, and commodity chemicals. There are a variety of metal initiators known, with one of the most attractive being that derived from reaction of WCl6 with an aryl amine and an alkyl aluminium halide. In collaboration with Sasol Technology UK we are probing the underpinning chemistry surrounding these types of tungsten system, which are believed to involve high oxidation state imido derivatives [9], and developing initiators with enhanced performance (Scheme 5).
Research Methodologies
Many of the synthetic procedures used by the group involve working with air- and moisture-sensitive materials that are handled using inert atmosphere glove-box and Schlenk line techniques. Routine use is made of many spectroscopic and analytical techniques including UV-vis, IR, and multinuclear NMR spectroscopies; mass spectrometry; gas chromatography; and X-ray crystallography (undertaken in collaboration with the group of Prof. Judith Howard). Throughout their work the group makes extensive use of 31P NMR spectroscopy, which lends itself very effectively to the in situ monitoring of reactions and makes a powerful diagnostic probe for compound identification and characterisation. Additionally, solid-state NMR is often used to provide further compositional and structural information (collaboration with Dr David Apperley and Fraser Markwell). To further support the group's synthetic chemistry theoretical/computational techniques are applied to determine and rationalize the structure, reactivity, and properties of the compounds prepared. In this area the group collaborate closely with Dr Mark Fox (Durham); Drs Jean-François Halet and Karine Costuas (Université de Rennes 1, France); and Drs Karinne Miqueu and Jean-Marc Sotiropoulos (Université de Pau et des Pays de l'Adour, France).
Catalyst testing often involves the manipulation of gases under pressure for which the group use dedicated autoclave systems and benefit from access to the Chemistry Department's unique high pressure chemistry facility. Recently, the group have designed and built a high-pressure loop-flow reactor for performing gas/liquid reactions at elevated pressures and, in particular, those involving homogeneous catalysts. The reactor can be used in one of two ways: for rapid/efficient catalyst screening (closed loop) or for undertaking preparative-scale reactions involving gaseous substrates (open loop). The system's small volume brings significant advantages in terms of safety and reactant/solvent economy. In collaboration with Cambridge Reactor Design (www.cambridgereactordesign.com) and the group of Prof. Graham Sandford (Durham), the group have started to explore pressurised gas/liquid reactions in flow regimes.
Selected References
[1] D. A. Smith, A. S. Batsanov, K. Costuas, R. Edge, D. C. Apperley, D. Collison, J.-F. Halet, J. A. K. Howard, P. W. Dyer, Angew. Chemie, Int. Ed., 2010, DOI: 10.1002/201003946.
[2] P. W. Dyer, J. Fawcett, M. J. Hanton, Organometallics, 2008, 27, 5082-5087.
[3] C. E. Anderson, A. S. Batsanov, P. W. Dyer, J. A. K. Howard, Dalton Trans., 2006, 5362-5378.
[4] P. W. Dyer, J. Fawcett, M. J. Hanton, J. Organomet. Chem., 2005, 690, 5264-5281.
[5] L. Baiget, A. S. Batsanov, P. W. Dyer, M. A. Fox, M. J. Hanton, J. A. K. Howard, P. K. Lane, S. Solomon,
Dalton Trans., 2008, 1043-1056.
[6] D. A. Smith, A. S. Batsanov, K. Miqueu, J-M. Sotiropoulos, D. C. Apperley, J. A. K. Howard, P. W. Dyer, Angew. Chem. Int. Ed., 2008, 47, 8674-8677.
[7] D. A. Smith, A. S. Batsanov, M. A. Fox, A. Beeby, D. C. Apperley, J. A. K. Howard, P. W. Dyer, Angew. Chemie, Int. Ed., 2009, 48, 9109-9113.
[8] C. E. Anderson, D. C. Apperley, A. S. Batsanov, P. W. Dyer, J. A. K. Howard, Dalton Trans., 2006, 4134-4145.
[9] W. R. H. Wright, A. S. Batsanov, J. A. K. Howard, R. P. Tooze, M. J. Hanton, P. W. Dyer, Dalton Trans., 2010, 39, 7038-7045.
Research interests
- Organometallic Chemistry
- Homogeneous Catalysis
- Organophosphorus Chemistry
Publications
Chapter in book
- An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass ConversionLi, L., Rowbotham, J. S., Greenwell, H. C., & Dyer, P. W. (2013). An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass Conversion. In S. L. Suib (Ed.), New and future developments in catalysis : catalytic biomass conversion. (pp. 173-208). Elsevier. https://doi.org/10.1016/b978-0-444-53878-9.00009-6
Journal Article
- Room Temperature Ethene to Propene (ETP) Tandem Catalysis using Single Crystalline Solid-State Molecular Pre-CatalystsAltus, K. M., Shi, Y., Probst, P., Heaton, J. H., Gyton, M. R., Lari, L., Buchmeiser, M. R., Dyer, P. W., & Weller, A. S. (2025). Room Temperature Ethene to Propene (ETP) Tandem Catalysis using Single Crystalline Solid-State Molecular Pre-Catalysts. Angewandte Chemie International Edition. Advance online publication, Article e202419923. https://doi.org/10.1002/anie.202419923
- Thermo-catalytic reforming pyrolysis of ensiled Saccharina latissima dominated macroalgal pellets for bioenergy productionKirby, M. E., Toop, T., Ouadi, M., McEvoy, L., Rolin, C., Inkster, R., Dyer, P. W., & Theodorou, M. K. (2024). Thermo-catalytic reforming pyrolysis of ensiled Saccharina latissima dominated macroalgal pellets for bioenergy production. Energy Conversion and Management: X, 24, Article 100692. https://doi.org/10.1016/j.ecmx.2024.100692
- Conversion of butanol to propene in flow: A triple dehydration, isomerisation and metathesis cascadeShi, Y., Weller, A. S., Blacker, A. J., & Dyer, P. W. (2022). Conversion of butanol to propene in flow: A triple dehydration, isomerisation and metathesis cascade. Catalysis Communications, 164, Article 106421. https://doi.org/10.1016/j.catcom.2022.106421
- Process-oriented approach towards catalyst design and optimisationAbbasi, M. R., Galvanin, F., Blacker, A. J., Sorensen, E., Shi, Y., Dyer, P. W., & Gavriilidis, A. (2022). Process-oriented approach towards catalyst design and optimisation. Catalysis Communications, 163, Article 106392. https://doi.org/10.1016/j.catcom.2021.106392
- Versatile, Cheap, Readily Modifiable Sample Delivery Method for Analysis of Air-/Moisture-Sensitive Samples Using Atmospheric Pressure Solids Analysis Probe Mass SpectrometryStrong, K. A., Stokes, P., Parker, D., Buckley, A. K., Mosely, J. A., Brodie, C. N., & Dyer, P. W. (2022). Versatile, Cheap, Readily Modifiable Sample Delivery Method for Analysis of Air-/Moisture-Sensitive Samples Using Atmospheric Pressure Solids Analysis Probe Mass Spectrometry. Analytical Chemistry, 94(32), 11315-11320. https://doi.org/10.1021/acs.analchem.2c02039
- Opening the Egg Box: NMR spectroscopic analysis of the interactions between s-block cations and kelp monosaccharidesRowbotham, J., Greenwell, C., & Dyer, P. W. (2021). Opening the Egg Box: NMR spectroscopic analysis of the interactions between s-block cations and kelp monosaccharides. Dalton Transactions, 50(38), 13246-13255. https://doi.org/10.1039/d0dt04375c
- Selective dimerisation of 1-hexene mediated by aluminium alkyl chloride-activated tungsten imido complexesDyer, P. W., Hanton, M. J., & Messinis, A. M. (2020). Selective dimerisation of 1-hexene mediated by aluminium alkyl chloride-activated tungsten imido complexes. Catalysis Science & Technology. Advance online publication. https://doi.org/10.1039/d0cy01863e
- Solution-state behaviour of algal mono-uronates evaluated by pure shift and compressive sampling NMR techniquesRowbotham, J. S., Aguilar, J. A., Kenwright, A. M., Greenwell, H. C., & Dyer, P. W. (2020). Solution-state behaviour of algal mono-uronates evaluated by pure shift and compressive sampling NMR techniques. Carbohydrate Research, 495, Article 108087. https://doi.org/10.1016/j.carres.2020.108087
- Additives boosting the performance of tungsten imido-mediated ethylene dimerization systems for industrial applicationMessinis, A. M., Wright, W. R., Hanton, M. J., & Dyer, P. W. (2020). Additives boosting the performance of tungsten imido-mediated ethylene dimerization systems for industrial application. Chemical Communications, 56(50), 6886-6889. https://doi.org/10.1039/d0cc03077e
- Activated niobium and tantalum imido complexes: from tuneable polymerization to selective ethylene dimerization systemsMessinis, A., Batsanov, A., Howard, J., Hanton, M., & Dyer, P. W. (2019). Activated niobium and tantalum imido complexes: from tuneable polymerization to selective ethylene dimerization systems. ChemCatChem, 11(6), 1756-1764. https://doi.org/10.1002/cctc.201801849
- Ketone Formation via Decarboxylation Reactions of Fatty Acids Using Solid Hydroxide/Oxide CatalystsSmith, B., Li, L., Perera-Solis, D., Gildea, L., Zholobenko, V., Dyer, P., & Greenwell, H. C. (2018). Ketone Formation via Decarboxylation Reactions of Fatty Acids Using Solid Hydroxide/Oxide Catalysts. Inorganics, 6(4), Article 121. https://doi.org/10.3390/inorganics6040121
- Bis(imido) tungsten complexes: efficient pre-catalysts for the homogeneous dimerization of ethyleneMessinis, A., Batsanov, A. S., Wright, W. R., Howard, J. A., Hanton, M. J., & Dyer, P. W. (2018). Bis(imido) tungsten complexes: efficient pre-catalysts for the homogeneous dimerization of ethylene. ACS Catalysis, 8(12), 11249-11263. https://doi.org/10.1021/acscatal.8b02202
- Exploration of Homogeneous Ethylene Dimerization Mediated by Tungsten Mono(imido) ComplexesMessinis, A. M., Wright, W. R., Batsanov, A. S., Howard, J. A., Hanton, M. J., & Dyer, P. W. (2018). Exploration of Homogeneous Ethylene Dimerization Mediated by Tungsten Mono(imido) Complexes. ACS Catalysis, 8(12), 11235-11248. https://doi.org/10.1021/acscatal.8b02201
- Species variation in the effects of dewatering treatment on macroalgaeGallagher, J. A., Turner, L. B., Adams, J. M., Barrento, S., Dyer, P. W., & Theodorou, M. K. (2018). Species variation in the effects of dewatering treatment on macroalgae. Journal of Applied Phycology, 30(4), 2305-2316. https://doi.org/10.1007/s10811-018-1420-7
- Biodiesel production via trans-esterification using Pseudomonas cepacia immobilized on cellulosic polyurethaneLi, L., Dyer, P. W., & Greenwell, H. C. (2018). Biodiesel production via trans-esterification using Pseudomonas cepacia immobilized on cellulosic polyurethane. ACS Omega, 3(6), 6804-6811. https://doi.org/10.1021/acsomega.8b00110
- The Role of Catalyst Support, Diluent and Co-Catalyst in Chromium-Mediated Heterogeneous Ethylene TrimerisationLamb, M., Apperley, D., Watson, M., & Dyer, P. (2018). The Role of Catalyst Support, Diluent and Co-Catalyst in Chromium-Mediated Heterogeneous Ethylene Trimerisation. Topics in Catalysis, 61(3-4), 213-224. https://doi.org/10.1007/s11244-018-0891-8
- Analysis of air-, moisture- and solvent-sensitive chemical compounds by mass spectrometry using an inert atmospheric pressure solids analysis probeMosely, J. A., Stokes, P., Parker, D., Dyer, P. W., & Messinis, A. M. (2017). Analysis of air-, moisture- and solvent-sensitive chemical compounds by mass spectrometry using an inert atmospheric pressure solids analysis probe. European Journal of Mass Spectrometry, 24(1), 74-80. https://doi.org/10.1177/1469066717732286
- Changes in higher heating value and ash content of seaweed during ensilingRedden, H., Milledge, J. J., Greenwell, H. C., Dyer, P. W., & Harvey, P. J. (2017). Changes in higher heating value and ash content of seaweed during ensiling. Journal of Applied Phycology, 29(2), 1037-1046. https://doi.org/10.1007/s10811-016-0975-4
- Dewatering treatments to increase dry matter content of the brown seaweed, kelp (Laminaria digitata ((Hudson) JV Lamouroux))Gallagher, J. A., Turner, L. B., Adams, J. M., Dyer, P. W., & Theodora, M. K. (2016). Dewatering treatments to increase dry matter content of the brown seaweed, kelp (Laminaria digitata ((Hudson) JV Lamouroux)). Bioresource Technology, 224, 662-669. https://doi.org/10.1016/j.biortech.2016.11.091
- Phosphanyl Methanimine (PCN) Ligands for the Selective Trimerization/Tetramerization of Ethylene with ChromiumRadcliffe, J. E., Batsanov, A. S., Smith, D. M., Scott, J. A., Dyer, P. W., & Hanton, M. J. (2015). Phosphanyl Methanimine (PCN) Ligands for the Selective Trimerization/Tetramerization of Ethylene with Chromium. ACS Catalysis, 5(12), 7095-7098. https://doi.org/10.1021/acscatal.5b02106
- Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed BiomassMilledge, J. J., Smith, B., Dyer, P. W., & Harvey, P. (2014). Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies, 7(11), 7194-7222. https://doi.org/10.3390/en7117194
- Combined DFT and experimental studies of C–C and C–X elimination reactions promoted by a chelating phosphine–alkene ligand: the key role of penta-coordinate PdIIEstévez, L., Tuxworth, L. W., Sotiropoulos, J., Dyer, P. W. D., & Miqueu, K. (2014). Combined DFT and experimental studies of C–C and C–X elimination reactions promoted by a chelating phosphine–alkene ligand: the key role of penta-coordinate PdII. Dalton Transactions, 43(29), 11165-11179. https://doi.org/10.1039/c4dt00340c
- Phosphine-alkene ligand-mediated alkyl-alkyl and alkyl-halide elimination processes from palladium(II)Tuxworth, L., Baiget, L., Phanopoulos, A., Metters, O. J., Batsanov, A. S., Fox, M. A., Howard, J. A., & Dyer, P. W. (2012). Phosphine-alkene ligand-mediated alkyl-alkyl and alkyl-halide elimination processes from palladium(II). Chemical Communications, 48(84), 10413-10415. https://doi.org/10.1039/c2cc35623f
- Exploiting Non-Innocent Ligands to Prepare Masked Palladium(0) ComplexesSmith, D. A., Batsanov, A., Costuas, K., Edge, R., Apperley, D., Collison, D., Halet, J., Howard, J., & Dyer, P. (2010). Exploiting Non-Innocent Ligands to Prepare Masked Palladium(0) Complexes. Angewandte Chemie International Edition, 49(39), 7194-7198. https://doi.org/10.1002/anie.201003946
- From Cyclic Iminophosphoranes to -Conjugated MaterialsSmith, D., Batsanov, A., Fox, M., Beeby, A., Apperley, D., Howard, J., & Dyer, P. (2009). From Cyclic Iminophosphoranes to -Conjugated Materials. Angewandte Chemie International Edition, 48(48), 9109-9113. https://doi.org/10.1002/anie.200904219
- Bridging M-Cl Bonds with Ambiphilic Phosphine–Borane LigandsBontemps, S., Bouhadir, G., Apperley, D., Dyer, P., Miqueu, K., & Bourissou, D. (2009). Bridging M-Cl Bonds with Ambiphilic Phosphine–Borane Ligands. Chemistry - An Asian Journal, 4(3), 428-435. https://doi.org/10.1002/asia.200800388
- Ambiphilic diphosphine-borane ligands: Metal -> borane interactions within isoelectronic complexes of rhodium, platinum and palladiumBontemps, S., Sircoglou, M., Bouhadir, G., Puschmann, H., Howard, J. A., Dyer, P. W., Miqueu, K., & Bourissou, D. (2008). Ambiphilic diphosphine-borane ligands: Metal -> borane interactions within isoelectronic complexes of rhodium, platinum and palladium. Chemistry - A European Journal, 14(2), 731-740. https://doi.org/10.1002/chem.200701027
- A Truly Multifunctional Heterocycle: Iminophosphorane, N,P Chelate, and DihydropyridineSmith, D., Batsanov, A., Miqueu, K., Sotiropoulos, J., Apperley, D., Howard, J., & Dyer, P. (2008). A Truly Multifunctional Heterocycle: Iminophosphorane, N,P Chelate, and Dihydropyridine. Angewandte Chemie International Edition, 47(45), 8674-8677. https://doi.org/10.1002/anie.200803373
- Rigid N-Phosphino Guanidine P,N Ligands and Their Use in Nickel-Catalyzed Ethylene OligomerizationDyer, P., Fawcett, J., & Hanton, M. (2008). Rigid N-Phosphino Guanidine P,N Ligands and Their Use in Nickel-Catalyzed Ethylene Oligomerization. Organometallics, 27(19), 5082-5087. https://doi.org/10.1021/om8005933
- Chelating N-pyrrolylphosphino-N-arylaldimine ligands : synthesis, ligand behaviour and applications in catalysis.Anderson, C., Batsanov, A., Dyer, P., Fawcett, J., & Howard, J. (2006). Chelating N-pyrrolylphosphino-N-arylaldimine ligands : synthesis, ligand behaviour and applications in catalysis. Dalton Transactions, 45, 5362-5378. https://doi.org/10.1039/b611652c
- Concise syntheses of tridentate PNE ligands and their coordination chemistry with palladium(II): a solution- and solid-state studyAnderson, C., Apperley, D., Batsanov, A., Dyer, P., & Howard, J. (2006). Concise syntheses of tridentate PNE ligands and their coordination chemistry with palladium(II): a solution- and solid-state study. Dalton Transactions, 2006(34), 4134-4145. https://doi.org/10.1039/b605995c
- Diphenylphosphino(phenyl pyridin-2-yl methylene)amine palladium(II) complexes: Chemoselective alkene hydrocarboxylation initiatorsDyer, P., Fawcett, J., & Hanton, M. (2005). Diphenylphosphino(phenyl pyridin-2-yl methylene)amine palladium(II) complexes: Chemoselective alkene hydrocarboxylation initiators. Journal of Organometallic Chemistry, 690(23), 5264-5281. https://doi.org/10.1016/j.jorganchem.2005.04.032
- Sterically-controlled regioselective para-substitutions of anilineDyer, P., Fawcett, J., Griffith, G., Hanton, M., Olivier, C., Patterson, A., & Suhard, S. (2005). Sterically-controlled regioselective para-substitutions of aniline. Chemical Communications, 2005(30), 3835-3837. https://doi.org/10.1039/b506824j
- The rise of organophosphorus derivatives in pi-conjugated materials chemistryHissler, M., Dyer, P., & Reau, R. (2005). The rise of organophosphorus derivatives in pi-conjugated materials chemistry. New Aspects in Phosphorus Chemistry V, 250, 127-163.
- The oxidative addition of a chlorophosphine to Pd-0: formation and characterisation of a 42-electron triangulo palladium clusterDyer, P., Fawcett, J., Hanton, M., Mingos, D., & Williamson, A. (2004). The oxidative addition of a chlorophosphine to Pd-0: formation and characterisation of a 42-electron triangulo palladium cluster. Dalton Transactions, 2004(16), 2400-2401. https://doi.org/10.1039/B408519A
- Exploring the coordination chemistry and reactivity of dialkyamino- and bis(dialkylamino)-phosphines in the coordination sphere of metalsDyer, P., Fawcett, J., Hanton, M., Kemmitt, R., Padda, R., & Singh, N. (2003). Exploring the coordination chemistry and reactivity of dialkyamino- and bis(dialkylamino)-phosphines in the coordination sphere of metals. Dalton Transactions, 2003(1), 104-113. https://doi.org/10.1039/b208886j
- Stable (Aryl(phosphino)carbenes: New ligands for transition metalsDespagnet, E., Miqueu, K., Gornitzka, H., Dyer, P., Bourissou, D., & Bertrand, G. (2002). Stable (Aryl(phosphino)carbenes: New ligands for transition metals. Journal of the American Chemical Society, 124, 11834-11835. https://doi.org/10.1021/ja027201i
- The 'one-pot' syntheses of alpha,alpha'-diphosphino-substituted imines: a unique reaction of bulky bis(dialkylamino)chlorophosphinesBaceiredo, A., Bertrand, G., Dyer, P., Fawcett, J., Griep-Raming, N., Guerret, O., Hanton, M., Russell, D., & Williamson, A. (2001). The ’one-pot’ syntheses of alpha,alpha’-diphosphino-substituted imines: a unique reaction of bulky bis(dialkylamino)chlorophosphines. New Journal of Chemistry, 25, 591-596. https://doi.org/10.1039/b009867l
- Synthesis and reactivity of group 6 and 10 complexes of the bis(dialkylaminophosphanyl)imine iPrN=C[CH2P(NiPr2)2]2Dahan, F., Dyer, P., Hanton, M., Jones, M., Mingos, D., White, C., Williams, D., & Williamson, A. (n.d.). Synthesis and reactivity of group 6 and 10 complexes of the bis(dialkylaminophosphanyl)imine iPrN=C[CH2P(NiPr2)2]2. European Journal of Inorganic Chemistry, 732-742.

