Staff profile
Professor Robert Pal
Professor

| Affiliation | Telephone |
|---|---|
| Professor in the Department of Chemistry | +44 (0) 191 33 42102 |
Biography
Circularly Polarised Luminescence Spectroscopy and Microscopy
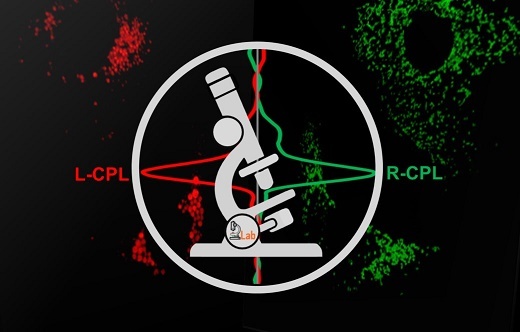
The molecular machinery of life is founded on chiral building blocks, but no experimental technique is currently available to distinguish or monitor chiral systems in live cell bio-imaging studies. We set out to harness the phenomenon of Circularly Polarised Luminescence (CPL), where different enantiomers of the same chemical entity produce different handedness (left or right) of emitted light.
In 2016, we developed a pioneering prototype Circularly Polarised Luminescence (CPL) epifluorescence microscope that selectively observes the emission of right and left-handed circularly polarised light (Chem. Commun., 2016, 52, 13349). Almost all our instrumental development can be attributed to our unique position where we have led the way in the development of responsive optical bio-imaging probes based on lanthanide luminescence (Nature Rev. Chem, 2021, 5, 109).
Our most recently developed all solid-state SS-CPL spectrometer is a paradigm shift in CPL spectroscopy (Nat. Commun., 2020, 11, 1676), a technique that has been hindered in the last 50 years by its inherent limitations due to its unchanged design that prevented its use and widespread application. For the first time, our novel CPL spectrometer not only allows time-resolved CPL spectroscopy to be exploited, but when coupled to a femtosecond laser as a proof-of-concept, we successfully recorded the first-ever (2PE) CPL spectrum using two photon-excitation.
In 2022 we have published our ground-breaking article detailing blueprints and live cell applications of the world’s first CPL Laser Scanning Confocal Microscope (CPL-LSCM) (Nat. Commun., 2022, 13, 553) capable of simultaneous enantioselective differential chiroptical contrast based live-cell imaging of endogenous and engineered CPL-active cellular probes.
We hope that this milestone in microscopy and CPL research empowers the multidisciplinary imaging community to embark on a journey to the uncharted and study chiral interactions on a sub-cellular level in a new (chiral) light.
Molecular Nanomachines
Using a new generation of light activated cell type specific molecular nanomachines we have demonstrated the use of molecular mechanical action to open cellular membranes and lipid bilayers and expedite cell death in a fully controlled manner. The efficacy of this method using single photon excitation in the UV domain was demonstrated and has been recently published in Nature (2017, 548, 567-572).
Our overarching future aim is to extend our study beyond in vitro applications. Using UV light to activate these molecular machines in vivo has signification limitations associated with shallow tissue penetration and potential UV damage. In order to overcome this we propose to extend our research into the two-photon-, near-infrared activated domains.
Our vision for the future is to develop and validate a series of light-activated molecular machines to selectively target cancerous cells in the human body and safely eradicate them, in order to pave the way to the development of a fundamentally new photodynamic therapy protocol combining cell type and metabolic/morphological state specific uni-molecular nanomachines and biologically safe near infra-red activation. Once fully developed and validated it could be potentially adopted as a new form of extremely high 3D optical precision, facile and non-invasive Type V photodynamic therapy to eliminate the need of currently used highly invasive surgical or radiotherapeutic procedures that often harmful to administer.
https://www.youtube.com/watch?v=UE_Zh8XUDuE
Member of the Royal Microscopical Society

https://www.rms.org.uk/

Robert Pal grew up in Hungary and graduated from KLTE University of Debrecen, in 2004. Once completing his undergraduate studies he has moved to Durham to start a Ph.D with Professor David Parker on Responsive Luminescent Lanthanide systems. Completing his Ph.D in late 2007 he began to work as a Postdoctoral researcher within the Parker group, also working closely with Professor Andrew Beeby, moving away from organic chemistry towards bio-physical chemistry, spectroscopy and microscopy. In 2014 he has been awarded with a prestigious University Research Fellowship from the Royal Society to study the Development and Chemical Application of Phase Modulation Nanoscopy.

Robert is also the Technical Director of a successful University spin out company, FScan Ltd, which has developed lanthanide technology to be used as part of a novel test for Prostate Cancer detection that is currently part of an ongoing clincial trial.
He has also founded PB Spectroscopy limited alongside Prof. Andrew Beeby, a company that is dedicated for the development of miniaturised and affordable spectroscopic instrumentation and solutions.
Research Interests

RP is a physical chemist working on the border of organic chemistry and biophysics with expertise in lanthanide based sensors and cellular probes. In recent years he has focused his main research interest on innovation in the development of bespoke optical instrumentation, notably for high resolution, affordable microscopy and in portable optical spectroscopy for emission and circular polarised luminescence. In addition he also strives to capitalise on his new research interest in targeted light activated molecular nanomachines (Nature 2017).
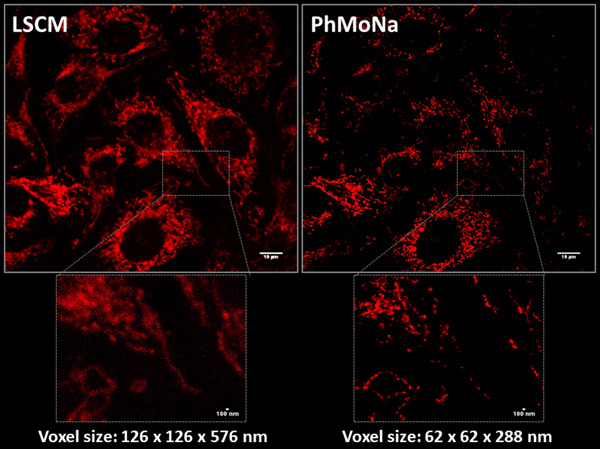
Super-resolution Microscopy or ‘Nanoscopy’
'A picture is worth more than 100 words'
In the field of optical fluorescence microscopy, many researchers have dedicated their entire scientific carrier to develop better and improved cellular stains or associated microscopy techniques and equipment, pushing the boundaries of both signal detection and resolution. However, the intrinsic resolution of fluorescence microscopy is limited by diffraction, which determines the extension of the focused light emitted by a point source object, in other words the point spread function (PSF). In recent years, renewed have efforts emerged in optical microscopy and associated life sciences to break through the optical diffraction barrier and visualize even smaller parts of the ‘living’ cell in ‘super-resolution’. Governed by Abbé’s law (1873) the highest achievable theoretical spatial resolution (d) of a given experimental setup is dictated by the lowest applied excitation light (λexc) d = λexc./2NA (NA: numerical aperture of objective) promoting maximal achievable resolutions of ~200 nm. However, much of the fundamental biology of the cell occurs below this threshold; hence breaking this limit will allow the wide multidisciplinary scientific community to look deeper in higher resolution.
Ever since the step-changing invention of Confocal Microscopy by Minsky in 1953, attention has turned towards the development of new optical (hardware) and computer (software) based methodologies, revolutionising the way in which we investigate nanostructures. Recent years seen many refined and evolved variants of ‘Super-resolution’ techniques by improvements applied either or both to the illumination or detection process leading to improvement in both precision and resolution. No need to say this combined multidisciplinary effort have recently been recognised with the Nobel-prize awarded in Chemistry in 2014. But these new, still fundamentally diffraction limited techniques all have drawbacks too, such as limitation of suitable stains, disruptively high laser powers, slow/limited imaging speed, poor SNR due to loss of information and specialized expensive and bulky instrumentation.
Taking the above into account, the obvious conclusion for me is to introduce novel user friendly resolution enhancing methods (both hard- and sofware) into existing microscope platforms, on what several members of our research group is working on.
Research interests
- Circularly Polarised Luminescence Spectroscopy and Microscopy
- Optical Microscopy
- Optical Spectroscopy
- Photophysics
- Light Activated Molecular Nanomachines
Esteem Indicators
- 2022: Director of Space and Infrastructure:
- 2019: Chair of Public Engagement, Department of Chemistry:
- 2019: Chair of Ethics, Department of Chemistry:
- 2018: Member of the Royal Microscopical Society: https://www.rms.org.uk/
- 2018: Outreach and Public Engagement:
- University public engagement champion in Durham and nationally. Outreach and Academic demonstrator for PonDetective, Origins of Elements, Meet the Universe, Light Entertainment, Spectroscopy in a Suitcase and the highly successful and nationally renowned Lights, Colours, Camera, Action! in several high impact public science events (Science Museum Lates, Royal Soc. Summer Sci. Exhibition, British Science Week, FameLab/Chelthenham Science Festival: in excess of 10,000 people/year attendance). Outreach representative of business and research translational related activies, guest lecturer on UK based CDT projects and scientific advisory duties for the Royal Society and BBC. Royal Society Young People’s Book Prize judge 2017.
Publications
Journal Article
- Two‐photon excitation of bright diaza[4]helicenes for isotropic and circularly polarized emissionFabri, B., De Rosa, D. F., Black, D. J., Mucci, R., Krimovs, A., Pal, R., & Lacour, J. (2025). Two‐photon excitation of bright diaza[4]helicenes for isotropic and circularly polarized emission. Chemistry: A European Journal, 31(32), Article e202501212. https://doi.org/10.1002/chem.202501212
- A Bis‐Perylene Diimide Macrocycle Chiroptical SwitchHartmann, D., Penty, S. E., Zwijnenburg, M. A., Pal, R., & Barendt, T. A. (2025). A Bis‐Perylene Diimide Macrocycle Chiroptical Switch. Angewandte Chemie, 64(15), Article e202501122. https://doi.org/10.1002/ange.202501122
- A switch-on luminescent europium(III) probe for selective and time-resolved detection of adenosine diphosphate (ADP)Bodman, S. E., Stachelek, P., Rehman, U., Plasser, F., Pal, R., & Butler, S. J. (2025). A switch-on luminescent europium(III) probe for selective and time-resolved detection of adenosine diphosphate (ADP). Chemical Science. Advance online publication. https://doi.org/10.1039/d4sc07188c
- Exerting control of self-assembly pathways via morphological switching and patterning in amino-acid-based benzene-1,3,5-tricarboxamide conjugatesSavyasachi, A. J., Kotova, O., Luis, E. T., Lynes, A. D., Mills, S., Bright, S. A., McManus, G. J., Möbius, M. E., Williams, D. C., Pal, R., Boland, J. J., & Gunnlaugsson, T. (2025). Exerting control of self-assembly pathways via morphological switching and patterning in amino-acid-based benzene-1,3,5-tricarboxamide conjugates. Chem, 11(2), Article 102321. https://doi.org/10.1016/j.chempr.2024.09.020
- Self-assembling Depsipeptides on Aggregation-Induced Emission Luminogens: A New Way to Create Programmable Nanovesicles and Soft Nanocarriers.Hernando-Muñoz, C., Revilla-Cuesta, A., Abajo-Cuadrado, I., Andreini, C., Torroba, T., Busto, N., Fernández, D., Perdomo, G., Acosta, G., Royo, M., Gutierrez Reguera, J., Spinello, A., Barone, G., Black, D., & Pal, R. (2025). Self-assembling Depsipeptides on Aggregation-Induced Emission Luminogens: A New Way to Create Programmable Nanovesicles and Soft Nanocarriers. ACS Applied Materials and Interfaces, 17(6), 8751-10192. https://doi.org/10.1021/acsami.4c19123
- Autoclave reactor synthesis of upconversion nanoparticles, unreported variables, and safety considerationsMcGonigle, R., Glasgow, J., Houston, C., Cameron, I., Homann, C., Black, D. J., Pal, R., & MacKenzie, L. E. (2025). Autoclave reactor synthesis of upconversion nanoparticles, unreported variables, and safety considerations. Communications Chemistry, 8, Article 36. https://doi.org/10.1038/s42004-025-01415-3
- Ligand Chirality Transfer from Solution State to the Crystalline Self‐Assemblies in Circularly Polarized Luminescence (CPL) Active Lanthanide SystemsCaffrey, D. F., Gorai, T., Rawson, B., Martínez‐Calvo, M., Kitchen, J. A., Murray, N. S., Kotova, O., Comby, S., Peacock, R. D., Stachelek, P., Pal, R., & Gunnlaugsson, T. (2024). Ligand Chirality Transfer from Solution State to the Crystalline Self‐Assemblies in Circularly Polarized Luminescence (CPL) Active Lanthanide Systems. Advanced Science, 11(18), Article 2307448. https://doi.org/10.1002/advs.202307448
- A Chirally Locked Bis-perylene Diimide Macrocycle: Consequences for Chiral Self-Assembly and Circularly Polarized LuminescencePenty, S. E., Orton, G. R., Black, D., Pal, R., Zwijnenburg, M. A., & Barendt, T. A. (2024). A Chirally Locked Bis-perylene Diimide Macrocycle: Consequences for Chiral Self-Assembly and Circularly Polarized Luminescence. Journal of the American Chemical Society, 146(8), 5470-5479. https://doi.org/10.1021/jacs.3c13191
- Distinguishing Molecular Mechanical Action from Photothermal and Photodynamic BehaviorBeckham, J. L., Bradford, T. S., Ayala-Orozco, C., Santos, A. L., Arnold, D., van Venrooy, A. R., García-López, V., Pal, R., & Tour, J. M. (2024). Distinguishing Molecular Mechanical Action from Photothermal and Photodynamic Behavior. Advanced Materials, 36(7), Article 2306669. https://doi.org/10.1002/adma.202306669
- Unlocking same‐sign CPL: solvent effects on spectral form and racemisation kinetics in nine‐coordinate chiral europium(III) complexesDe Rosa, D. F., Starck, M., Parker, D., & Pal, R. (2024). Unlocking same‐sign CPL: solvent effects on spectral form and racemisation kinetics in nine‐coordinate chiral europium(III) complexes. Chemistry - A European Journal, 30(9), Article e202303227. https://doi.org/10.1002/chem.202303227
- Circularly Polarized Luminescence Bioimaging Using Chiral BODIPYs: A Model Scaffold for Advancing Unprecedented CPL Microscopy Using Small Full-Organic Probes.Stachelek, P., Serrano-Buitrago, S., Maroto, B. L., Pal, R., & de la Moya, S. (2024). Circularly Polarized Luminescence Bioimaging Using Chiral BODIPYs: A Model Scaffold for Advancing Unprecedented CPL Microscopy Using Small Full-Organic Probes. ACS Applied Materials and Interfaces, 16(49). https://doi.org/10.1021/acsami.4c14127
- Two-Photon Circularly Polarized Luminescence of Chiral Eu ComplexesWillis, O. G., Petri, F., De Rosa, D. F., Mandoli, A., Pal, R., Zinna, F., & Di Bari, L. (2023). Two-Photon Circularly Polarized Luminescence of Chiral Eu Complexes. Journal of the American Chemical Society, 145(46), 25170-25176. https://doi.org/10.1021/jacs.3c05957
- 1‐Azathioxanthone Appended Lanthanide(III) DO3A Complexes That Luminesce Following Excitation at 405 nm**Gundorff Nielsen, L., Ravnsborg Hansen, A. K., Stachelek, P., Pal, R., & Just Sørensen, T. (2023). 1‐Azathioxanthone Appended Lanthanide(III) DO3A Complexes That Luminesce Following Excitation at 405 nm**. European Journal of Inorganic Chemistry, 26(24), Article e202300245. https://doi.org/10.1002/ejic.202300245
- Influence of polyvinylpyrrolidone (PVP) in the synthesis of luminescent NaYF4:Yb,Er upconversion nanoparticlesBirch, R., Bruckbauer, J., Gajewska, M., Cios, G., Pal, R., & MacKenzie, L. E. (2023). Influence of polyvinylpyrrolidone (PVP) in the synthesis of luminescent NaYF4:Yb,Er upconversion nanoparticles. Methods and Applications in Fluorescence, 11(3), Article 034001. https://doi.org/10.1088/2050-6120/acd837
- Rapid handheld time-resolved circularly polarised luminescence photography camera for life and material sciencesDe Rosa, D. F., Brook, P., Black, D. J., & Pal, R. (2023). Rapid handheld time-resolved circularly polarised luminescence photography camera for life and material sciences. Nature Communications, 14, Article 1537. https://doi.org/10.1038/s41467-023-37329-8
- Hierarchical self-assembly in an RNA-based coordination polymer hydrogelEl-Zubir, O., Rojas Martinez, P., Dura, G., Doherty, C., Cucinotta, F., Mackenzie, L. E., Pal, R., Horrocks, B. R., & Houlton, A. (2023). Hierarchical self-assembly in an RNA-based coordination polymer hydrogel. Dalton Transactions, 52(17), 5545-5551. https://doi.org/10.1039/d3dt00634d
- Near-Infrared Circularly Polarised Luminescence from Helically Extended Chiral N,N,O,O -Boron Chelated DipyrromethenesAlgoazy, N., Clarke, R. G., Penfold, T. J., Waddell, P. G., Probert, M. R., Aerts, R., Herrebout, W., Stachelek, P., Pal, R., Hall, M. J., & Knight, J. G. (2022). Near-Infrared Circularly Polarised Luminescence from Helically Extended Chiral N,N,O,O -Boron Chelated Dipyrromethenes. ChemPhotoChem, 6(9), Article e202200090. https://doi.org/10.1002/cptc.202200090
- The Pink Box: Exclusive Homochiral Aromatic Stacking in a Bis-perylene Diimide MacrocyclePenty, S. E., Zwijnenburg, M. A., Orton, G. R., Stachelek, P., Pal, R., Xie, Y., Griffin, S. L., & Barendt, T. A. (2022). The Pink Box: Exclusive Homochiral Aromatic Stacking in a Bis-perylene Diimide Macrocycle. Journal of the American Chemical Society, 144(27), 12290-12298. https://doi.org/10.1021/jacs.2c03531
- BINOL blocks as accessible triplet state modulators in BODIPY dyesJiménez, J., Prieto-Montero, R., Serrano, S., Stachelek, P., Rebollar, E., Maroto, B. L., Moreno, F., Martinez-Martinez, V., Pal, R., García-Moreno, I., & de la Moya, S. (2022). BINOL blocks as accessible triplet state modulators in BODIPY dyes. Chemical Communications, 58(44), 6385-6388. https://doi.org/10.1039/d2cc00991a
- Lanthanide luminescence from supramolecular hydrogels consisting of bio-conjugated picolinic-acid-based guanosine quadruplexesKotova, O., O’Reilly, C., Barwich, S. T., Mackenzie, L. E., Lynes, A. D., Savyasachi, A. J., Ruether, M., Pal, R., Möbius, M. E., & Gunnlaugsson, T. (2022). Lanthanide luminescence from supramolecular hydrogels consisting of bio-conjugated picolinic-acid-based guanosine quadruplexes. Chem, 8(5), 1395-1414. https://doi.org/10.1016/j.chempr.2022.01.015
- Low-temperature open-air synthesis of PVP-coated NaYF4:Yb,Er,Mn upconversion nanoparticles with strong red emissionMacKenzie, L. E., Alvarez-Ruiz, D., & Pal, R. (2022). Low-temperature open-air synthesis of PVP-coated NaYF4:Yb,Er,Mn upconversion nanoparticles with strong red emission. Royal Society Open Science, 9(1). https://doi.org/10.1098/rsos.211508
- Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactionsStachelek, P., MacKenzie, L., Parker, D., & Pal, R. (2022). Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions. Nature Communications, 13(1), Article 533. https://doi.org/10.1038/s41467-022-28220-z
- Striking solvent dependence of total emission and circularly polarised luminescence in coordinatively saturated chiral europium complexes: solvation significantly perturbs the ligand fieldFradgley, J. D., Frawley, A. T., Pal, R., & Parker, D. (2021). Striking solvent dependence of total emission and circularly polarised luminescence in coordinatively saturated chiral europium complexes: solvation significantly perturbs the ligand field. Physical Chemistry Chemical Physics, 23(19), 11479-11487. https://doi.org/10.1039/d1cp01686e
- Synthesis and Evaluation of Europium Complexes that Switch On Luminescence in Lysosomes of Living CellsStarck, M., Fradgley, J. D., Pal, R., Zwier, J. M., Lamarque, L., & Parker, D. (2021). Synthesis and Evaluation of Europium Complexes that Switch On Luminescence in Lysosomes of Living Cells. Chemistry - A European Journal, 27(2), 766-777. https://doi.org/10.1002/chem.202003992
- Applying TADF Emitters in Bioimaging and Sensing—A Novel Approach Using Liposomes for Encapsulation and Cellular UptakeSmith, P. O., Black, D. J., Pal, R., Avó, J., Dias, F. B., Linthwaite, V. L., Cann, M. J., & Pålsson, L. (2021). Applying TADF Emitters in Bioimaging and Sensing—A Novel Approach Using Liposomes for Encapsulation and Cellular Uptake. Frontiers in Chemistry, 9, Article 743928. https://doi.org/10.3389/fchem.2021.743928
- Targeted Luminescent Europium Peptide Conjugates: Comparative Analysis Using Maleimide and para-Nitropyridyl Linkages for Organelle StainingStarck, M., Fradgley, J. D., Di Vita, S., Mosely, J., Pal, R., & Parker, D. (2020). Targeted Luminescent Europium Peptide Conjugates: Comparative Analysis Using Maleimide and para-Nitropyridyl Linkages for Organelle Staining. Bioconjugate Chemistry, 31(2), 229-240. https://doi.org/10.1021/acs.bioconjchem.9b00735
- Development of tris-cyclometalated iridium complexes for cellular imaging through structural modificationMeksawangwong, S., Gohil, B., Punyain, W., Pal, R., & Kielar, F. (2020). Development of tris-cyclometalated iridium complexes for cellular imaging through structural modification. Inorganica Chimica Acta, 508, Article 119609. https://doi.org/10.1016/j.ica.2020.119609
- Anticancer Ruthenium Complexes with HDAC Isoform SelectivityCross, J., Blower, T., Kingdon, A., Pal, R., Picton, D., & Walton, J. (2020). Anticancer Ruthenium Complexes with HDAC Isoform Selectivity. Molecules, 25(10), Article 2383. https://doi.org/10.3390/molecules25102383
- Molecular Nanomachines Can Destroy Tissue or Kill Multicellular EukaryotesGunasekera, R. S., Galbadage, T., Ayala-Orozco, C., Liu, D., García-López, V., Troutman, B. E., Tour, J. J., Pal, R., Krishnan, S., Cirillo, J. D., & Tour, J. M. (2020). Molecular Nanomachines Can Destroy Tissue or Kill Multicellular Eukaryotes. ACS Applied Materials and Interfaces, 12(12), 13657-13670. https://doi.org/10.1021/acsami.9b22595
- Time-resolved luminescence detection of peroxynitrite using a reactivity-based lanthanide probeBreen, C., Pal, R., Elsegood, M. R., Teat, S. J., Iza, F., Wende, K., Buckley, B. R., & Butler, S. J. (2020). Time-resolved luminescence detection of peroxynitrite using a reactivity-based lanthanide probe. Chemical Science, 11(12), 3164-3170. https://doi.org/10.1039/c9sc06053g
- Visible-Light-Activated Molecular Nanomachines Kill Pancreatic Cancer CellsAyala Orozco, C., Liu, D., Li, Y., Alemany, L. B., Pal, R., Krishnan, S., & Tour, J. M. (2020). Visible-Light-Activated Molecular Nanomachines Kill Pancreatic Cancer Cells. ACS Applied Materials and Interfaces, 12(1), 410-417. https://doi.org/10.1021/acsami.9b21497
- Three-dimensional data capture and analysis of intact eye lenses evidences emmetropia-associated changes and strain-dependent differences in epithelial cell organizationKalligeraki, A. A., Isted, A., Pal, R., Saunter, C., Girkin, J., Jarrin, M., Uwineza, A., Obara, B., & Quinlan, R. A. (2020). Three-dimensional data capture and analysis of intact eye lenses evidences emmetropia-associated changes and strain-dependent differences in epithelial cell organization. Scientific Reports, 10, Article 16898. https://doi.org/10.1038/s41598-020-73625-9
- π‐Expanded Thioxanthones ‐ Engineering the Triplet Level of Thioxanthone Sensitizers for Lanthanide‐Based Luminescent Probes with Visible ExcitationDansholm, C. N., Junker, A. K. R., Nielsen, L. G., Kofod, N., Pal, R., & Sørensen, T. J. (2019). π‐Expanded Thioxanthones ‐ Engineering the Triplet Level of Thioxanthone Sensitizers for Lanthanide‐Based Luminescent Probes with Visible Excitation. ChemPlusChem, 84(12), 1778-1788. https://doi.org/10.1002/cplu.201900309
- Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to MeropenemGalbadage, T., Liu, D., Alemany, L. B., Pal, R., Tour, J. M., Gunasekera, R. S., & Cirillo, J. D. (2019). Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to Meropenem. ACS Nano, 13(12), 14377-14387. https://doi.org/10.1021/acsnano.9b07836
- Excitation modulation of Eu:BPEPC based complexes as low-energy reference standards for circularly polarised luminescence (CPL)Starck, M., MacKenzie, L. E., Batsanov, A. S., Parker, D., & Pal, R. (2019). Excitation modulation of Eu:BPEPC based complexes as low-energy reference standards for circularly polarised luminescence (CPL). Chemical Communications, 55(94), 14115-14118. https://doi.org/10.1039/c9cc07290j
- Synthesis and investigation of a tris-cyclometalated iridium complex bearing a single quarternary ammonium groupMeksawangwong, S., Gohil, B., Punyain, W., Pal, R., & Kielar, F. (2019). Synthesis and investigation of a tris-cyclometalated iridium complex bearing a single quarternary ammonium group. Inorganica Chimica Acta, 497, Article 119066. https://doi.org/10.1016/j.ica.2019.119066
- Near Infrared Light Activates Molecular Nanomachines to Drill Into and Kill CellsLiu, D., García-López, V., Gunasekera, R. S., Greer Nilewski, L., Alemany, L. B., Aliyan, A., Jin, T., Wang, G., Tour, J. M., & Pal, R. (2019). Near Infrared Light Activates Molecular Nanomachines to Drill Into and Kill Cells. ACS Nano, 13(6), 6813-6823. https://doi.org/10.1021/acsnano.9b01556
- Holographic projection for multi-spot structured illumination microscopyWard, E., & Pal, R. (2019). Holographic projection for multi-spot structured illumination microscopy. Journal of Microscopy, 274(2), 114-120. https://doi.org/10.1111/jmi.12787
- In Vitro and in Cellulo Sensing of Transition Metals Using Time-Resolved Fluorescence Spectroscopy and MicroscopyPal, R., Barker, A. C., Hummel, D., & Pålsson, L. (2019). In Vitro and in Cellulo Sensing of Transition Metals Using Time-Resolved Fluorescence Spectroscopy and Microscopy. Journal of Fluorescence, 29(1), 255-263. https://doi.org/10.1007/s10895-018-2335-z
- Interaction of Nucleotides with a Trinuclear Terbium(III)–Dizinc(II) Complex: Efficient Sensitization of Terbium Luminescence by Guanosine Monophosphate and Application to Real-Time Monitoring of Phosphodiesterase ActivityAulsebrook, M. L., Starck, M., Grace, M. R., Graham, B., Thordarson, P., Pal, R., & Tuck, K. L. (2019). Interaction of Nucleotides with a Trinuclear Terbium(III)–Dizinc(II) Complex: Efficient Sensitization of Terbium Luminescence by Guanosine Monophosphate and Application to Real-Time Monitoring of Phosphodiesterase Activity. Inorganic Chemistry, 58(1), 495-505. https://doi.org/10.1021/acs.inorgchem.8b02731
- Highly Linearized Twisted Iridium(III) ComplexesDavidson, R., Hsu, Y., Griffiths, G. C., Li, C., Yufit, D., Pal, R., & Beeby, A. (2018). Highly Linearized Twisted Iridium(III) Complexes. Inorganic Chemistry, 57(22), 14450-14462. https://doi.org/10.1021/acs.inorgchem.8b02818
- Selective signalling of glyphosate in water using europium luminescenceJennings, L. B., Shuvaev, S., Fox, M. A., Pal, R., & Parker, D. (2018). Selective signalling of glyphosate in water using europium luminescence. Dalton Transactions, 47(45), 16145-16154. https://doi.org/10.1039/c8dt03823f
- Cationic Europium Complexes for Visualizing Fluctuations in Mitochondrial ATP Levels in Living CellsMailhot, R., Traviss-Pollard, T., Pal, R., & Butler, S. J. (2018). Cationic Europium Complexes for Visualizing Fluctuations in Mitochondrial ATP Levels in Living Cells. Chemistry - A European Journal, 24(42), 10745-10755. https://doi.org/10.1002/chem.201801008
- On the antibacterial activity of azacarboxylate ligands: lowered metal ion affinities for bis-amide derivatives of EDTA do not mean reduced activityMulla, R. S., Beecroft, M. S., Pal, R., Aguilar, J., Pitarch-Jarque, J., García‐España, E., Lurie-Luke, E., Sharples, G., & Williams, J. (2018). On the antibacterial activity of azacarboxylate ligands: lowered metal ion affinities for bis-amide derivatives of EDTA do not mean reduced activity. Chemistry - A European Journal, 24(28), 7137-7148. https://doi.org/10.1002/chem.201800026
- Enhancing multi-spot structured illumination microscopy with fluorescence differenceWard, E. N., Torkelsen, F. H., & Pal, R. (2018). Enhancing multi-spot structured illumination microscopy with fluorescence difference. Royal Society Open Science, 5(3), Article 171336. https://doi.org/10.1098/rsos.171336
- Enantioselective cellular localisation of europium(iii) coordination complexesFrawley, A. T., Linford, H., Starck, M., Pal, R., & Parker, D. (2018). Enantioselective cellular localisation of europium(iii) coordination complexes. Chemical Science, 9(4), 1042-1049. https://doi.org/10.1039/c7sc04422d
- Chiral transcription in self-assembled tetrahedral Eu4L6 chiral cages displaying sizable circularly polarized luminescenceYeung, C., Yim, K., Wong, H., Pal, R., Lo, W., Yan, S., Yee-Man Wong, M., Yufit, D., Smiles, D. E., McCormick, L. J., Teat, S. J., Shuh, D. K., Wong, W., & Law, G. (2017). Chiral transcription in self-assembled tetrahedral Eu4L6 chiral cages displaying sizable circularly polarized luminescence. Nature Communications, 8(1), Article 1128. https://doi.org/10.1038/s41467-017-01025-1
- Molecular machines open cell membranesGarcía-López, V., Chen, F., Nilewski, L. G., Duret, G., Aliyan, A., Kolomeisky, A. B., Robinson, J. T., Wang, G., Pal, R., & Tour, J. M. (2017). Molecular machines open cell membranes. Nature, 548(7669), 567-572. https://doi.org/10.1038/nature23657
- Circularly Polarised Luminescence from Helically Chiral “Confused” N,N,O,C-Boron-Chelated Dipyrromethenes (BODIPYs)Clarke, R., Ho, K. L., Alsimaree, A. A., Woodford, O. J., Waddell, P. G., Bogaerts, J., Herrebout, W., Knight, J. G., Pal, R., Penfold, T. J., & Hall, M. J. (2017). Circularly Polarised Luminescence from Helically Chiral “Confused” N,N,O,C-Boron-Chelated Dipyrromethenes (BODIPYs). ChemPhotoChem., 1(11), 513-517. https://doi.org/10.1002/cptc.201700106
- Image scanning microscopy: an overviewWard, E., & Pal, R. (2017). Image scanning microscopy: an overview. Journal of Microscopy, 266(2), 221-228. https://doi.org/10.1111/jmi.12534
- A tight tunable range for Ni(II) sensing and buffering in cellsFoster, A., Pernil, R., Patterson, C., Scott, A., Pålsson, L., Pal, R., Cummins, I., Chivers, P., Pohl, E., & Robinson, N. (2017). A tight tunable range for Ni(II) sensing and buffering in cells. Nature Chemical Biology, 13(4), 409-414. https://doi.org/10.1038/nchembio.2310
- Induced Europium Circularly Polarised Luminescence Monitors Reversible Drug Binding to Native α1-Acid GlycoproteinJennings, L., Waters, R., Pal, R., & Parker, D. (2017). Induced Europium Circularly Polarised Luminescence Monitors Reversible Drug Binding to Native α1-Acid Glycoprotein. ChemMedChem, 12(3), 271-277. https://doi.org/10.1002/cmdc.201600571
- Very bright, enantiopure europium(III) complexes allow time-gated chiral contrast imagingFrawley, A., Pal, D., & Parker, D. (2016). Very bright, enantiopure europium(III) complexes allow time-gated chiral contrast imaging. Chemical Communications, 52(91), 13349-13352. https://doi.org/10.1039/c6cc07313a
- Novel aminoalkyl tris-cyclometalated iridium complexes as cellular stainsSansee, A., Meksawangwong, S., Chainok, K., Franz, K., Gal, M., Palsson, L. O., Puniyan, W., Traiphol, R., Pal, R., & Kielar, F. (2016). Novel aminoalkyl tris-cyclometalated iridium complexes as cellular stains. Dalton Transactions, 45(43), 17420-17430. https://doi.org/10.1039/c6dt02776h
- Anisotropic lanthanide-based nano-clusters for imaging applicationsYang, X., Wang, S., King, T. L., Kerr, C. J., Blanchet, C., Svergun, D., Pal, R., Beeby, A., Vadivelu, J., Brown, K. A., Jones, R. A., Zhang, L., & Huang, S. (2016). Anisotropic lanthanide-based nano-clusters for imaging applications. Faraday Discussions, 191, 465-479. https://doi.org/10.1039/c6fd00018e
- New Class of Bright and Highly Stable Chiral Cyclen Europium Complexes for Circularly Polarized Luminescence ApplicationsDai, L., Lo, W., Coates, I. D., Pal, R., & Law, G. (2016). New Class of Bright and Highly Stable Chiral Cyclen Europium Complexes for Circularly Polarized Luminescence Applications. Inorganic Chemistry, 55(17), 9065-9070. https://doi.org/10.1021/acs.inorgchem.6b01546
- Induced europium CPL for the selective signalling of phosphorylated amino-acids and O-phosphorylated hexapeptidesNeil, E., Pal, R., Fox, M., & Parker, D. (2016). Induced europium CPL for the selective signalling of phosphorylated amino-acids and O-phosphorylated hexapeptides. Dalton Transactions, 45(20), 8355-8366. https://doi.org/10.1039/c6dt01212d
- Structural Control of Cell Permeability with Highly Emissive Europium(III) Complexes Permits Different Microscopy ApplicationsStarck, M., Pal, R., & Parker, D. (2016). Structural Control of Cell Permeability with Highly Emissive Europium(III) Complexes Permits Different Microscopy Applications. Chemistry - A European Journal, 22(2), 570-580. https://doi.org/10.1002/chem.201504103
- Wavelength-dependent optoacoustic imaging probes for NMDA receptor visualisationSim, N., Gottschalk, S., Pal, R., Delbianco, M., Degtyarik, D., Razansky, D., Westmeyer, G., Ntziachristos, V., Parker, D., & Mishra, A. (2015). Wavelength-dependent optoacoustic imaging probes for NMDA receptor visualisation. Chemical Communications, 51(82), 15149-15152. https://doi.org/10.1039/c5cc06277b
- Chiral probe development for circularly polarised luminescence: comparative study of structural factors determining the degree of induced CPL with four heptacoordinate europium(III) complexesNeil, E., Fox, M., Pal, R., Pålsson, L., O’Sullivan, B., & Parker, D. (2015). Chiral probe development for circularly polarised luminescence: comparative study of structural factors determining the degree of induced CPL with four heptacoordinate europium(III) complexes. Dalton Transactions, 44(33), 14937-14951. https://doi.org/10.1039/c5dt02358k
- Future challenges: general discussionJemmis, E., Aravamudhan, S., Arunan, E., Shahi, A., Hunt, N., Schnedermann, C., Helliwell, J. R., Ashfold, M., Goswami, H. P., Nenov, A., Deckert, V., Chowdhury, P. R., Ghiggino, K., Miller, R. D., Goswami, D., Junge, W., Howard, J., Tominaga, K., van Driel, T. B., … Mukamel, S. (2015). Future challenges: general discussion. Faraday Discussions, 177, 517-545. https://doi.org/10.1039/c5fd90019k
- Phase modulation nanoscopy: a simple approach to enhanced optical resolutionPal, R. (2015). Phase modulation nanoscopy: a simple approach to enhanced optical resolution. Faraday Discussions, 177, 507-515. https://doi.org/10.1039/c4fd00158c
- EuroTracker® dyes : design, synthesis, structure and photophysical properties of very bright europium complexes and their use in bioassays and cellular optical imagingButler, S., Delbianco, M., Lamarque, L., McMahon, B., Neil, E., Pal, R., Parker, D., Walton, J., & Zwier, J. (2015). EuroTracker® dyes : design, synthesis, structure and photophysical properties of very bright europium complexes and their use in bioassays and cellular optical imaging. Dalton Transactions, 44(11), 4791-4803. https://doi.org/10.1039/c4dt02785j
- Time and Space resolved Methods: general discussionZanni, M., E D, J., Aravamudhan, S., Pallipurath, A., Arunan, E., Schnedermann, C., Mishra, A. K., Warren, M., Hirst, J. D., John, F., Pal, R., Helliwell, J. R., Moirangthem, K., Chakraborty, S., Dijkstra, A. G., Roy Chowdhury, P., Ghiggino, K., Miller, R. D., Meech, S., … Goswami, D. (2015). Time and Space resolved Methods: general discussion. Faraday Discussions, 177, 263-292. https://doi.org/10.1039/c5fd90017d
- Magnetic resonance and optical imaging probes for NMDA receptors on the cell surface of neurons: synthesis and evaluation in celluloSim, N., Pal, R., Parker, D., Engelmann, J., Mishra, A., & Gottschalk, S. (2014). Magnetic resonance and optical imaging probes for NMDA receptors on the cell surface of neurons: synthesis and evaluation in cellulo. Organic and Biomolecular Chemistry, 12(46), 9389-9404. https://doi.org/10.1039/c4ob01848f
- Simple and versatile modifications allowing time gated spectral acquisition, imaging and lifetime profiling on conventional wide-field microscopesPal, R., & Beeby, A. (2014). Simple and versatile modifications allowing time gated spectral acquisition, imaging and lifetime profiling on conventional wide-field microscopes. Methods and Applications in Fluorescence, 2(3), Article 037001. https://doi.org/10.1088/2050-6120/2/3/037001
- Induced circularly polarized luminescence arising from anion or protein binding to racemic emissive lanthanide complexesCarr, R., Puckrin, R., McMahon, B. K., Pal, R., Parker, D., & Pålsson, L. (2014). Induced circularly polarized luminescence arising from anion or protein binding to racemic emissive lanthanide complexes. Methods and Applications in Fluorescence, 2(2), Article 024007. https://doi.org/10.1088/2050-6120/2/2/024007
- EuroTracker dyes: highly emissive europium complexes as alternative organelle stains for live cell imagingButler, S. J., Lamarque, L., Pal, R., & Parker, D. (2014). EuroTracker dyes: highly emissive europium complexes as alternative organelle stains for live cell imaging. Chemical Science, 2014(5), 1750-1756. https://doi.org/10.1039/c3sc53056f
- Utility of tris(4-bromopyridyl) europium complexes as versatile intermediates in the divergent synthesis of emissive chiral probesButler, S. J., Delbianco, M., Evans, N. H., Frawley, A. T., Pal, R., Parker, D., Puckrin, R. S., & Yufit, D. S. (2014). Utility of tris(4-bromopyridyl) europium complexes as versatile intermediates in the divergent synthesis of emissive chiral probes. Dalton Transactions, 43(15), 5721-5730. https://doi.org/10.1039/c4dt00253a
- Complete stereocontrol in the synthesis of macrocyclic lanthanide complexes: direct formation of enantiopure systems for circularly polarised luminescence applications.Evans, N. H., Carr, R., Delbianco, M., Pal, R., Yufit, D. S., & Parker, D. (2013). Complete stereocontrol in the synthesis of macrocyclic lanthanide complexes: direct formation of enantiopure systems for circularly polarised luminescence applications. Dalton Transactions, 42(44), 15610-6. https://doi.org/10.1039/c3dt52425f
- A bright and responsive europium probe for determination of pH change within the endoplasmic reticulum of living cells.McMahon, B. K., Pal, R., & Parker, D. (2013). A bright and responsive europium probe for determination of pH change within the endoplasmic reticulum of living cells. Chemical Communications, 49(47), 5363-5365. https://doi.org/10.1039/c3cc42308e
- Very bright europium complexes that stain cellular mitochondria.Walton, J., Bourdoll, A., Butler, S., Soulie, M., Delbianco, M., McMahon, B., Pal, R., Puschmann, H., Zwier, J., Lamarque, L., Maury, O., Andraud, C., & Parker, D. (2013). Very bright europium complexes that stain cellular mitochondria. Chemical Communications, 49(16), 1600-1602. https://doi.org/10.1039/c2cc35247h
- Bright Mono-aqua Europium Complexes Based on Triazacyclononane That Bind Anions Reversibly and Permeate Cells EfficientlyButler, S. J., McMahon, B. K., Pal, R., Parker, D., & Walton, J. W. (2013). Bright Mono-aqua Europium Complexes Based on Triazacyclononane That Bind Anions Reversibly and Permeate Cells Efficiently. Chemistry - A European Journal, 19(29), 9511-9517. https://doi.org/10.1002/chem.201301273
- Responsive MR-imaging probes for N-methyl-D-aspartate receptors and direct visualisation of the cell-surface receptors by optical microscopySim, N., Gottschalk, S., Pal, R., Engelmann, J., Parker, D., & Mishra, A. (2013). Responsive MR-imaging probes for N-methyl-D-aspartate receptors and direct visualisation of the cell-surface receptors by optical microscopy. Chemical Science, 4(8), 3148-3153. https://doi.org/10.1039/c3sc50903f
- Lanthanide Complexes Formed with the Tri- and Tetraacetate Derivatives of Bis(aminomethyl)phosphinic Acid: Equilibrium, Kinetic and NMR Spectroscopic StudiesTircso, G., Kalman, F. K., Pal, R., Banyai, I., Varga, T. R., Kiraly, R., Lazar, I., Quebatte, L., Merbach, A. E., Toth, E., & Bruecher, E. (2012). Lanthanide Complexes Formed with the Tri- and Tetraacetate Derivatives of Bis(aminomethyl)phosphinic Acid: Equilibrium, Kinetic and NMR Spectroscopic Studies. European Journal of Inorganic Chemistry, 2012(12), 2062-2073. https://doi.org/10.1002/ejic.201101299
- Live cell imaging of lysosomal pH changes with pH responsive ratiometric lanthanide probesSmith, D. G., McMahon, B. K., Pal, R., & Parker, D. (2012). Live cell imaging of lysosomal pH changes with pH responsive ratiometric lanthanide probes. Chemical Communications, 48(68), 8520-8522. https://doi.org/10.1039/c2cc34267g
- Analysis of citrate in low-volume seminal fluid samples using a time-gated measurement of europium luminescencePal, R., Beeby, A., & Parker, D. (2011). Analysis of citrate in low-volume seminal fluid samples using a time-gated measurement of europium luminescence. Journal of Pharmaceutical and Biomedical Analysis, 56(2), 352-358. https://doi.org/10.1016/j.jpba.2011.05.023
- A europium luminescence assay of lactate and citrate in biological fluidsPal, R., Parker, D., & Costello, L. C. (2009). A europium luminescence assay of lactate and citrate in biological fluids. Organic and Biomolecular Chemistry, 7(8), 1525-1528. https://doi.org/10.1039/b901251f
- Cell-Penetrating Metal Complex Optical Probes: Targeted and Responsive Systems Based on Lanthanide LuminescenceMontgomery, C. P., Murray, B. S., New, E. J., Pal, R., & Parker, D. (2009). Cell-Penetrating Metal Complex Optical Probes: Targeted and Responsive Systems Based on Lanthanide Luminescence. Accounts of Chemical Research, 42(7), 925-937. https://doi.org/10.1021/ar800174z
- Responsive and reactive terbium complexes with an azaxanthone sensitiser and one naphthyl group: applications in ratiometric oxygen sensing in vitro and in regioselective cell killingLaw, G., Pal, R., Palsson, L. O., Parker, D., & Wong, K. (2009). Responsive and reactive terbium complexes with an azaxanthone sensitiser and one naphthyl group: applications in ratiometric oxygen sensing in vitro and in regioselective cell killing. Chemical Communications, 47, 7321-7323. https://doi.org/10.1039/b920222f
- A ratiometric optical imaging probe for intracellular pH based on modulation of europium emissionPal, R., & Parker, D. (2008). A ratiometric optical imaging probe for intracellular pH based on modulation of europium emission. Organic and Biomolecular Chemistry, 6(6), 1020-1033. https://doi.org/10.1039/b718993a
- Critical evaluation of five emissive europium(III) complexes as optical probes: correlation of cytotoxicity, anion and protein affinity with complex structure, stability and intracellular localisation profileMurray, B. S., New, E. J., Pal, R., & Parker, D. (2008). Critical evaluation of five emissive europium(III) complexes as optical probes: correlation of cytotoxicity, anion and protein affinity with complex structure, stability and intracellular localisation profile. Organic and Biomolecular Chemistry, 6(12), 2085-2094. https://doi.org/10.1039/b803895c
- Complexation properties of the Di-, Tri-, and tetraacetate derivatives of bis(aminomethyl)phosphinic acidTircso, G., Benyei, A., Kiraly, R., Lazar, I., Pal, R., & Brucher, E. (2007). Complexation properties of the Di-, Tri-, and tetraacetate derivatives of bis(aminomethyl)phosphinic acid. European Journal of Inorganic Chemistry, 2007(5), 701-713. https://doi.org/10.1002/ejic.200600891
- A single component ratiometric pH probe with long wavelength excitation of europium emissionPal, R., & Parker, D. (2007). A single component ratiometric pH probe with long wavelength excitation of europium emission. Chemical Communications, 2007(5), 474-476. https://doi.org/10.1039/b616665b
- Azaxanthones and azathioxanthones are effective sensitisers for europium and terbium luminescenceAtkinson, P., Findlay, K., Kielar, F., Pal, D., Parker, D., Poole, R., Puschmann, H., Richardson, S., Stenson, P., Thompson, A., & Yu, J. (2006). Azaxanthones and azathioxanthones are effective sensitisers for europium and terbium luminescence. Organic and Biomolecular Chemistry, 4(9), 1707-1722. https://doi.org/10.1039/b601357k
Other (Print)
- Microscopic visualisation of metabotropic glutamate receptors on the surface of living cells using bifunctional magnetic resonance imaging probesMishra, A., Mishra, R., Gottschalk, S., Pal, R., Sim, N., Engelmann, J., Goldberg, M., & Parker, D. (2014). Microscopic visualisation of metabotropic glutamate receptors on the surface of living cells using bifunctional magnetic resonance imaging probes (pp. 128-137). ACS chemical neuroscience.

