Staff profile
Dr Victoria Money
Senior Manager Strategy & Performance

| Affiliation | Telephone |
|---|---|
| Senior Manager Strategy & Performance in the Research and Innovation Services | See Team Contact |
Biography
Victoria Money completed her PhD studies in chemical crystallography under the supervision of Prof. Judith Howard at Durham University in 2004. She then moved into the area of protein crystallography working with Prof. Gideon Davies at the York Structural Biology Laboratory. In 2006 she was awarded a European Molecular Biology Organisation Long-Term Fellowship and spent two years in Auckland, New Zealand working in the laboratory of Prof. Ted Baker studying membrane proteins from Mycobacterium tuberculosis. Since her return to the UK and Durham in 2008, Victoria has worked on a variety of projects and in 2010 was awarded a Royal Society University Research Fellowship.
Research Interests
The research within the group focuses on the application of a variety of biophysical techniques, including X-ray crystallography, to the study of viral encoded proteins from highly infectious human and animal pathogens.
The Mononegavirales
The viral order, Mononegavirales comprises some of the most infectious pathogens known to humankind including measles, mumps, Newcastle disease (which infects poultry) and Ebola. It also contains the newly emergent zoonotic viruses Nipah and Hendra which have been identified by the World Health Organisation as being of concern with regard to their potential to cause devastating pandemics.
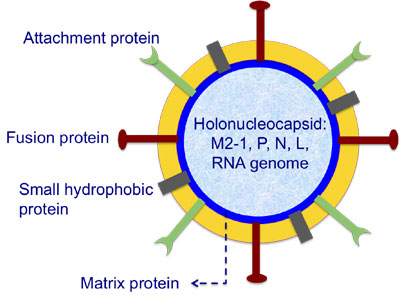
Figure 1: Schematic of the hRSV virus
Human respiratory syncytial virus (hRSV) is a member of the Mononegavirales (family Paramyxoviridae, subfamily Pneumovirinae), it is the single most important cause of lower respiratory tract infections, including pneumonia, in young children and infants. It is estimated that hRSV causes 64 million infections and 160, 000 deaths each year, worldwide. Despite the heavy social and economic burden imposed by this virus there is currently no effective anti-viral or vaccine available for vulnerable groups.
hRSV Matrix Protein (M), Figure 1, has a number of important roles within the viral replication cycle including the initiation of budding and the control of which host proteins are recruited to the viral envelope. In collaboration with Dr. John Sanderson (Chemistry) and Dr. Paul Yeo (Biomedical and Biological Sciences) we recently determined a high-resolution structure of the hRSV matrix protein, Figure 1. This was the first time the structure of a full-length matrix protein from this family of viruses had been determined. Examination of the structure of the M protein has provided a hypothesis for the way in which it binds to the viral envelope and how it controls curvature during the budding process. This hypothesis is currently being tested using model membranes
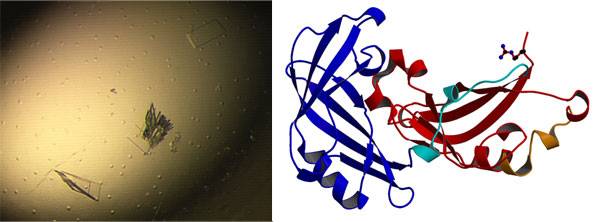
Figure 2: a) crystals of the matrix protein; b) cartoon of the matrix protein structure
Current research builds on this success to investigate the viral proteins involved in viral fusion, transcription regulation and pore formation in both hRSV and related viruses such as Hepatitis C. The results from this work will greatly improve our understanding of how these viruses behave and aid in the development of vaccines and drugs active against these important pathogens
Recent Publications
2011
Influence of Lipids on the Interfacial Disposition of Respiratory Syncytical Virus Matrix Protein, H. K. McPhee, J. L. Carlisle, A. Beeby, V. A. Money, S. M. D. Watson, R. Yeo, and J. M. Sanderson, Langmuir, 27 (2011), 304-311
2009
Surface features of a Mononegavirales Matrix protein indicate sites of membrane interaction, V. A. Money, H. K. McPhee, J. A. Mosely, J. M. Sanderson and R. P. Yeo, PNAS, 106 (2009), 4441-4446
The active site of a carbohydrate esterase displays divergent catalytic and noncatalytic binding functions, C. Montainer, V. A. Money, V. M. R. Pires, J. E. Flint, B. A. Pinheiro, A. Goyal, J. A. M. Prates, A. Izumi, H. Stålbrand. C. Morland, A. Cartmell, K. Kolenova, E. Topakas, E. J. Dodson, D. N. Bolam, G. J. Davies, C. M. G. A. Fontes and H. J. Gilbert, PLOS Biology, 7 (2009),
687-697
2008
The Clostridium cellulolyticum dockerin displays a dual binding mode for its cohesion partner, B. A. Pinheiro, M. R. Proctor, C. Martinez-Fleites, J. M. Prates, V. A. Money, G. J. Davies, E. A. Bayer, C. M. G. A. Fontes, H. P. Fierobe and H. J. Gilbert, J. Bio. Chem., 283 (2008), 18422-18430
Probing the beta-1,3:1,4 glucanase, CtLic26A, with a thio-oligosaccharide and enzyme varients, V. A. Money, A. Cartmell, C. I. P. D. Guerreiro, V. M. A. Ducros, C. M. G. A. Fontes, H. J. Gilbert and G. J. Davies, Org. Biomol. Chem, 6 (2008), 851-853
2007
D-Glucosylated Derivatives of Isofagomine and Noeuromycin and Their Potential as Inhibitors of β-Glycoside Hydrolases, P. J. Meloncelli, T. M. Gloster, V. A. Money, C. A. Tarling, G. J. Davies, S. G. Withers and R. V. Stick, Aust. J. Chem., 60 (2007), 549-565
Mannose foraging by Bacteroides thetaiotaomicron: Structure and specificity of the beta-mannosidase, Man2A, L. E. Tailford, V. A. Money, N. L. Smith, C. Dumon, G. J. Davies and H. J. Gilbert, J. Biol. Chem., 282 (2007), 11291-11299
Research interests
- Protein Crystallography
- Biophysical Techniques
Publications
Journal Article
- Influence of Lipids on the Interfacial Disposition of Respiratory Syncytical Virus Matrix ProteinMcPhee, H., Carlisle, J., Beeby, A., Money, V., Watson, M., Yeo, R., & Sanderson, J. (2011). Influence of Lipids on the Interfacial Disposition of Respiratory Syncytical Virus Matrix Protein. Langmuir, 27(1), 304-311. https://doi.org/10.1021/la104041n
- Surface features of a Mononegavirales matrix protein indicate sites of membrane interactionMoney, V. A., McPhee, H. K., Mosely, J. A., Sanderson, J. M., & Yeo, R. P. (2009). Surface features of a Mononegavirales matrix protein indicate sites of membrane interaction. Proceedings of the National Academy of Sciences, 106(11), 4441-4446. https://doi.org/10.1073/pnas.0805740106
- The spin-states and spin-crossover behaviour of iron(II) complexes of 2,6-dipyrazol-1-ylpyrazine derivativesElhaik, J., Money, V., Barrett, S., Kilner, C., Evans, I., & Halcrow, M. (2003). The spin-states and spin-crossover behaviour of iron(II) complexes of 2,6-dipyrazol-1-ylpyrazine derivatives. Dalton Transactions, 10, 2053-2060. https://doi.org/10.1039/b210368k

