Carbon dioxide interactions with nucleic acids in human health and sustainability
Carbon dioxide is one of the most important gases on Earth and an absolute requirement for life. The planet faces long term increases in atmospheric CO2, predicted to have a significant physiological impact on crops and with the potential for a chronic impact on physiology. Therefore, understanding CO2 biology is of pressing strategic importance. However, targets for CO2 sensing are relatively unknown. Identifying such targets is crucial for understanding the impact of CO2 on human health and in strategies to utilise CO2 as a feedstock in biofactories in sustainable industrial processes.
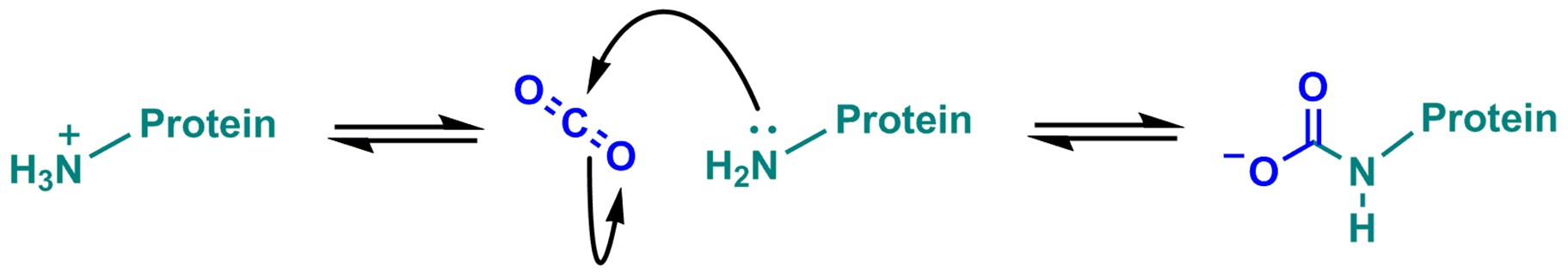
CO2 can spontaneously form a reversible protein post-translational modification through carbamylation of neutral N-terminal lysine e-amino groups (Figure 1).
We have previously developed a chemical proteomics technology for covalent trapping of protein carbamates that allows their identification by mass spectrometry (MS). This method has identified several high-profile CO2 protein targets. However, interactions between CO2 and nucleic acids are wholly unexplored. CO2 is known to regulate the transcriptome of all organisms examined suggesting a core conserved mechanism.
The aim of this project is to investigate how CO2 can interact with nucleic acids, the specific site of these interactions in a complex genome, and the functional relevance. The outcome will have a profound impact of our understanding of CO2 in human health and sustainability research.
The project will provide a core interdisciplinary skill set at the interface of chemistry and biology that includes biochemistry, molecular biology, genomics, organic chemistry, and physical chemistry. The project would suit students with either a chemical biology background or a background in the biosciences or chemistry and who wish to develop interdisciplinary skills to enhance their employability.
Please contact Prof Martin Cann (m.j.cann@durham.ac.uk) or Prof David Hodgson (d.r.w.hodgson@durham.ac.uk) for any questions regarding the project.
Funding Notes
Relevant publications
- Guillen-Garcia, A., Gibson, S.E.R., Jordan, C.J.C., Ramaswamy, V.K., Linthwaite, V.L., Bromley, E.H.C., Brown, A.P., Hodgson, D.R.W., Blower, T.R., Verlet, J.R.R., Degiacomi, M., Pålsson, L-O., Cann, M.J. (2022) Allophycocyanin A is a carbon dioxide receptor in the cyanobacterial phycobilisome. Nature Communications. 13: 5289 doi: 10.1038/s41467-022-32925-6.
- Blake, L.I. & Cann, M.J. (2022) Carbon dioxide and the carbamate post-translational modification. Frontiers in Molecular Biosciences. 9:825706. doi: 10.3389/fmolb.2022.825706
- Linthwaite, V.L., Pawloski, W., Pegg, H.B., Townsend, P.D., Thomas, M.J., So, V.K.H., Hodgson, D.R.W, Lorimer, G.H., Fushman, D., Cann, M.J. (2021) Ubiquitin is a carbon dioxide-binding protein. Science Advances. 7: eabi5507
- Linthwaite, V.L., Janus, J.M., Brown, A.P., Wong-Pascua, D., O’Donoghue, A.C., Porter, A., Treumann, A., Hodgson, D.R.W., Cann, M.J. (2018) The identification of carbon dioxide mediated protein post-translational modifications. Nature Communications 9:3092 | DOI: 10.1038/s41467-018-05475-z


/prod01/prodbucket01/media/durham-university/departments-/biosciences/83453-1-1595X1594.jpg)